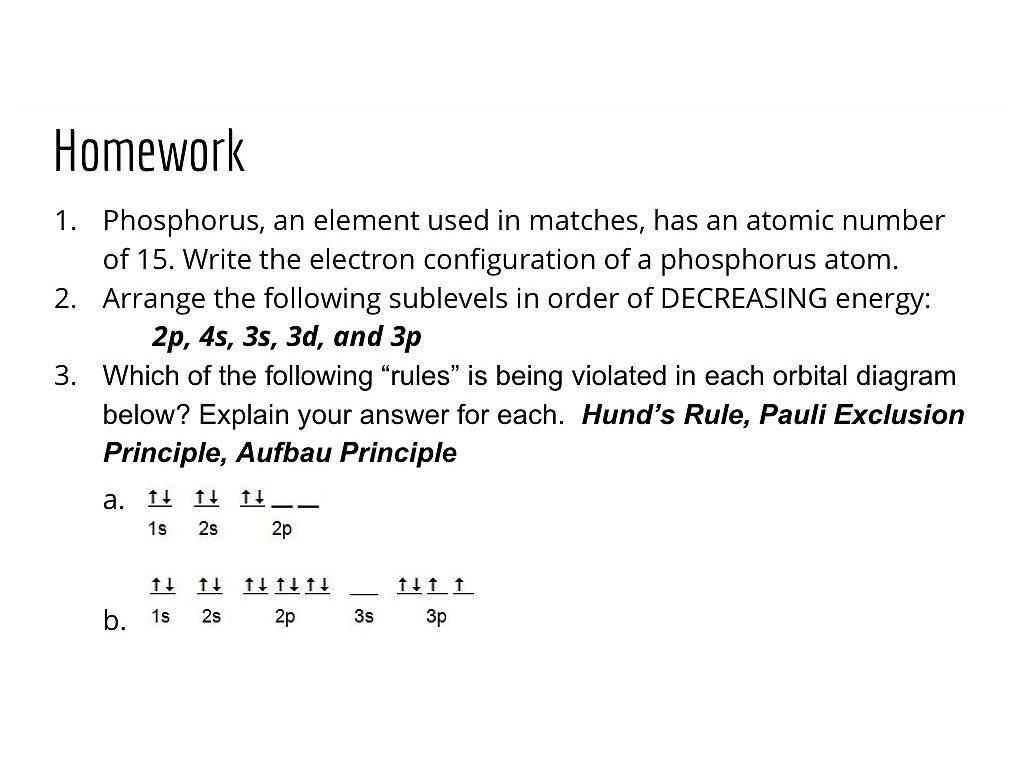
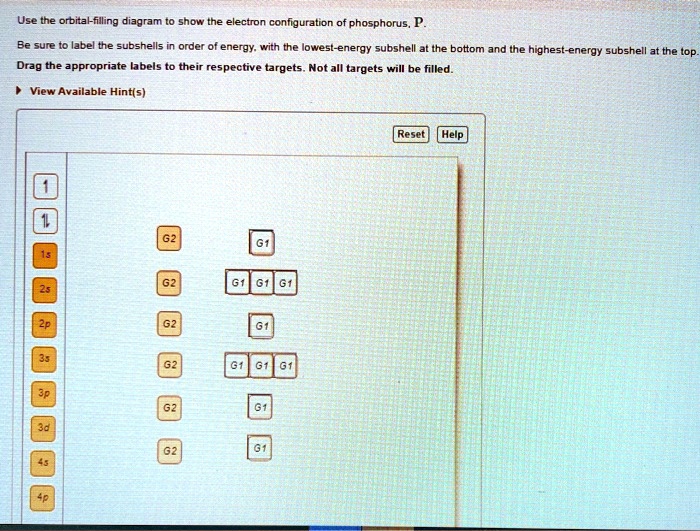
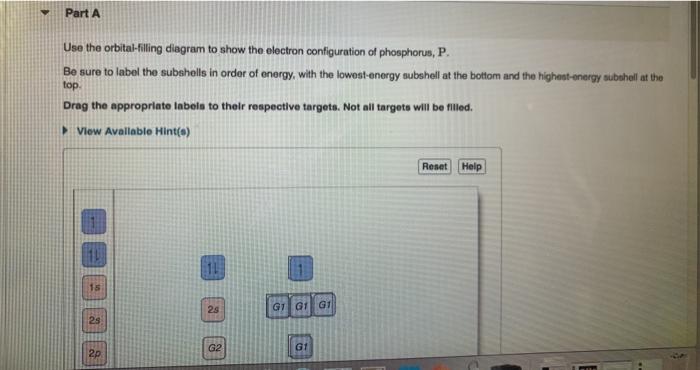
39 orbital diagram for phosphorus
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. PLEASE HELP! Write the full electron ... - Brainly.com PLEASE HELP! Write the full electron configuration for phosphorus, atomic symbol P, then draw an orbital box diagram that accounts for all of the electrons in phosphorus. You don't need to include the orbital box diagram as part of your answer. Based on your drawing, explain why phosphorus is either paramagnetic or diamagnetic.
Electron Configurations, Orbital Box Notation (M7Q7 ... The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct.

Orbital diagram for phosphorus
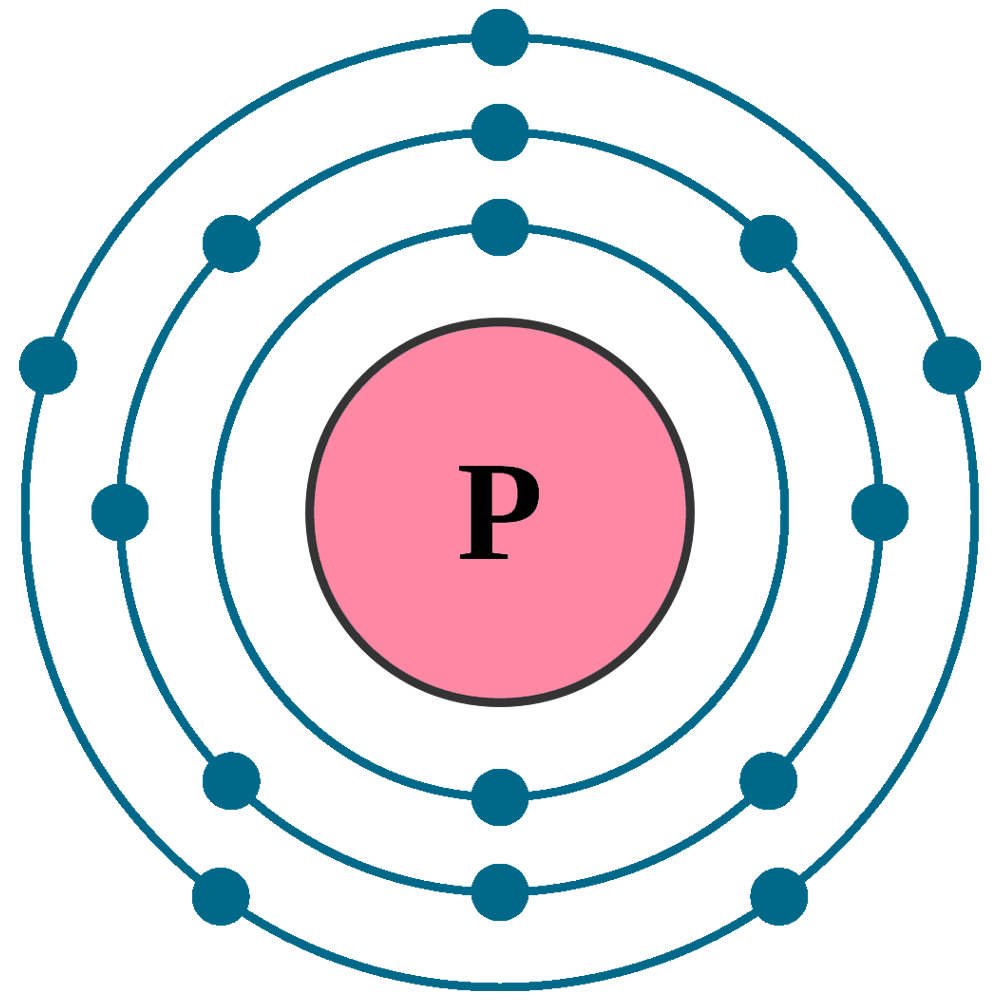
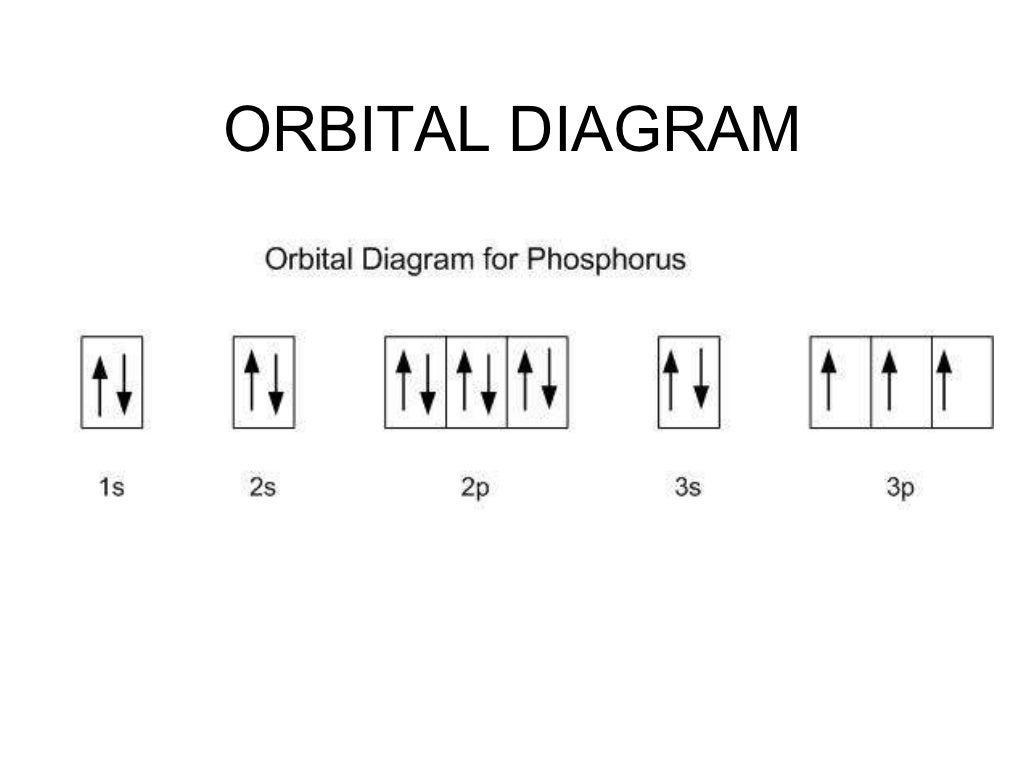
Phosphorus Orbital diagram, Electron configuration, and ... Orbital diagram for Phosphorus. The orbital diagram simply represents the arrangement of electrons in the different orbital of an atom, it uses an arrow to represent the electrons, every orbital(one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an atom. (1). PDF Which of the following is the correct orbital diagram for ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. What is the orbital diagram of phosphorus? - Rhumbarlv.com What is the orbital diagram of phosphorus? It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s and 3p. The outermost orbitals, 3s and 3p, have 5 electrons which are available for bonding with other atoms. The electronic configuration of phosphorus is 1s22s22p63s23p3 1 s 2 2 s 2 2 p 6 3 s ...
Orbital diagram for phosphorus. Quantum Numbers and Electron Configurations | Electronic ... What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Solution. The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . . What is the abbreviated electron configuration for phosphorus? One may also ask, what is the orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 ... Solved Fill in the orbital energy diagram for the | Chegg.com Question: Fill in the orbital energy diagram for the phosphorus. 3p 3s 2p 2s 1s A. A main group element with the valence electron configuration 4s is in periodic group It forms a monatomic ion with a charge of B. A main group element with the valence electron configuration 2s22p5 is in periodic group It forms a monatomic ion with a charge of. Draw and explain the orbital diagram for phosphorus ... Answer to: Draw and explain the orbital diagram for phosphorus. By signing up, you'll get thousands of step-by-step solutions to your homework...
Orbital Box Diagram Phosphorus - schematron.org A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital ... Zinc(Zn) electron configuration and orbital diagram The 3p orbital is now full. So, the next two electrons will enter the 4s orbital and the remaining ten electrons will enter the 3d orbital. Therefore, the zinc(Zn) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. How to write the orbital diagram for zinc(Zn)? PDF Phosphorus orbital energy diagram What is the correct orbital diagram for phosphorus. What is the orbital diagram for phosphorus. nortcele eroc eht sehctam noitarugifnoc esohw lobmys sag elbon eht htiw snortcele eroc eht secalper )thgir( noitarugifnoc nortcele detaiverbba-eroc A .latibro s2 eht ni ecaps gniniamer eht sllif nortcele htruof ehT .tcurtsnoc ot drawrofthgiarts si ... How do you write the orbital diagram for phosphate? | Socratic The whole point of that was to see how the oxygen orbital energies split up (and which were two-fold or three-fold degenerate). It was also to figure out which orbitals on phosphorus interact with which orbitals on the oxygen atoms. The resultant MO diagram was: Takeaways: This is only qualitative, so take it with a grain of salt.
Orbital diagram phosphorus? - Answers Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ... Phosphorus(P) electron configuration and orbital diagram Phosphorus(P) is the 15th element in the periodic table and its symbol is 'P'. The electron configuration of phosphorus and the orbital diagram is the main topic of this article. Also, valency and valence electrons of phosphorus, various reactions, and compound formation, bond formation have been discussed. Hopefully, after reading this ... PDF Electron Configurations and Orbital Diagrams key 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0 (you will never find an electron in the nucleus). 3. 2The electron configuration for phosphorus, written in core notation, is [Ne] 3s 3p 3. What two things does Hund's rule tell us Solved When completing the orbital diagram for the | Chegg.com When completing the orbital diagram for the element phosphorus, which of the following statements is correct? (3 points) There are five electrons in the n = 3 energy level. The 2p sublevel is not full. There are electrons in the 3d sublevel. There are no electrons in the 3s sublevel. There is one unpaired electron in the 3p sublevel.
How to Write the Orbital Diagram for Phosphorus (P) - YouTube To write the orbital diagram for the Phosphorus atom (P) first we need to write the electron configuration for just P. To do that we need to find the number...
Aufbau Diagram For Phosphorus The aufbau diagram shows the. The atomic number of phosphorus is Write the electron configuration of a phosphorus atom. 1s22s22p63s23p3. You can obtain correct electron configurations for the elements up to. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital.
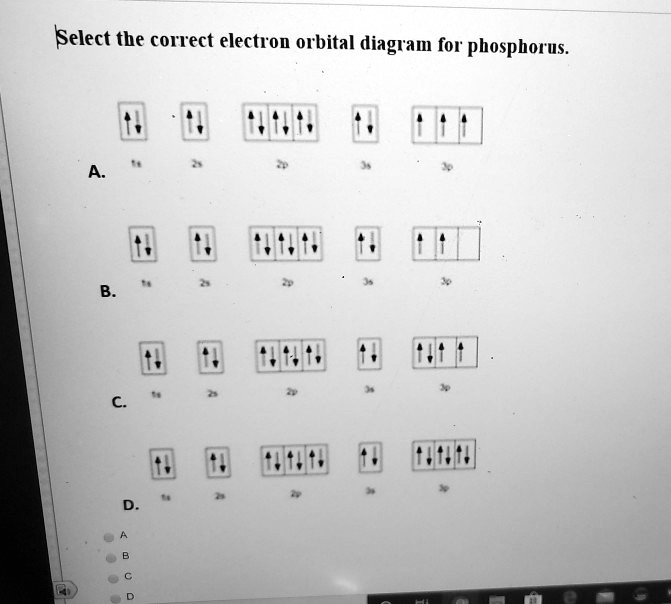
Answered: How many unpaired electrons are in the… | bartleby Question. 4) How many unpaired electrons are in the orbital diagram for phosphorus? Group of answer choices. A) 0. B) 6. C) 2. D) 3. E) 1. Expert Solution.
The orbital diagram of ground state Phosphorus atom has to ... The orbital diagram of ground state Phosphorus atom has to be written. Concept introduction: Pauli Exclusion Principle An orbital having a most two electrons and in this two electrons have opposite spins. Each orbital having no more than two electrons and similar spin is not allowed.
What is the orbital diagram for phosphorus? - AnswersToAll What is the orbital diagram for phosphorus? In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
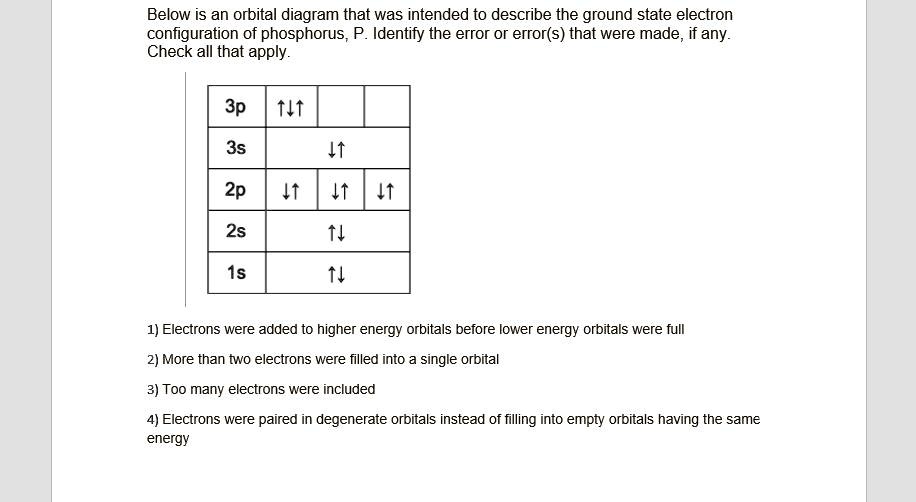
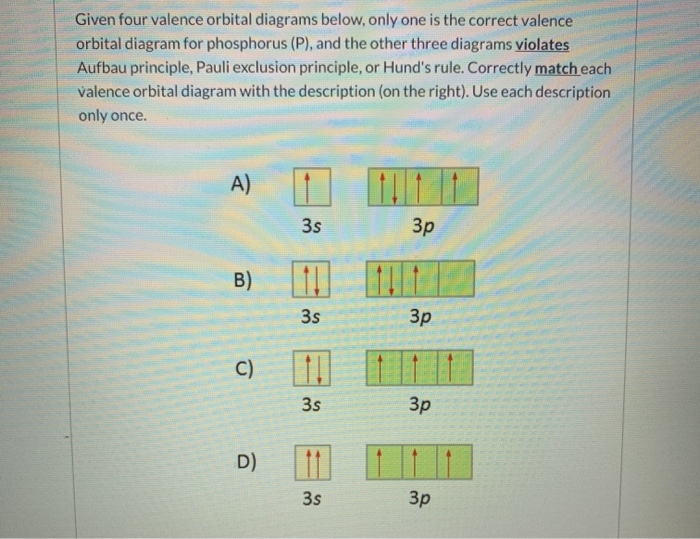
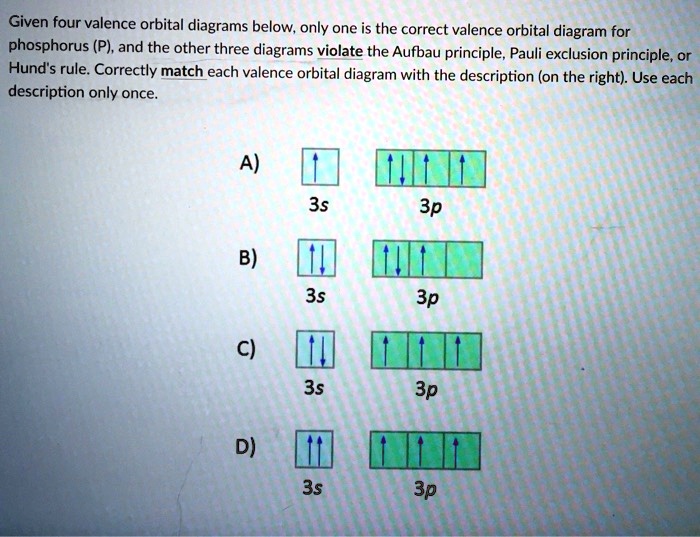
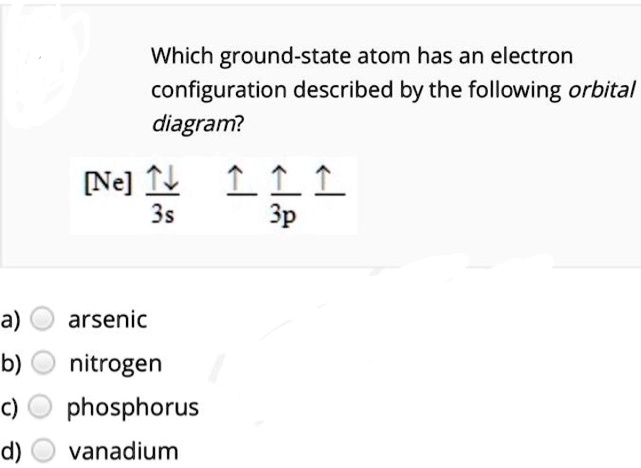
chem question 2.docx - Question 1 1 / 1 pts Given four ... Question 1 1 / 1 pts Given four valence orbital diagrams below, only one is the correct valence orbital diagram for phosphorus (P), and the other three diagrams violates Aufbau principle, Pauli exclusion principle, or Hund's rule. Correctly match each valence orbital diagram with the description (on the right). Use each description only once. Diagram A) Diagram B) Diagram C) It is not the ...
Phosphorus Orbital Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
Aufbau Diagram For Phosphorus - schematron.org Locate the nearest noble gas preceding phosphorus in the periodic table. Then subtract its number of electrons from those in phosphorus to obtain the number of valence electrons in phosphorus. Referring to Figure , draw an orbital diagram to represent those valence orbitals. In writing the electron configuration for Phosphorus the first two ...
Electron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ...
What is the orbital diagram of phosphorus? - Rhumbarlv.com What is the orbital diagram of phosphorus? It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s and 3p. The outermost orbitals, 3s and 3p, have 5 electrons which are available for bonding with other atoms. The electronic configuration of phosphorus is 1s22s22p63s23p3 1 s 2 2 s 2 2 p 6 3 s ...
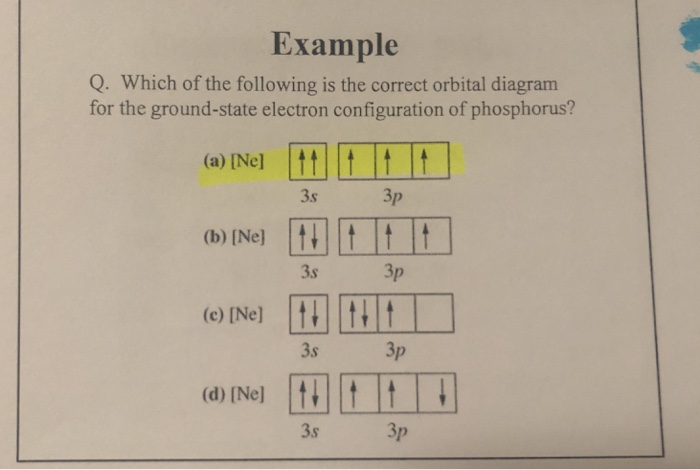
PDF Which of the following is the correct orbital diagram for ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Phosphorus Orbital diagram, Electron configuration, and ... Orbital diagram for Phosphorus. The orbital diagram simply represents the arrangement of electrons in the different orbital of an atom, it uses an arrow to represent the electrons, every orbital(one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an atom. (1).






















Comments
Post a Comment