43 orbital diagram for mg
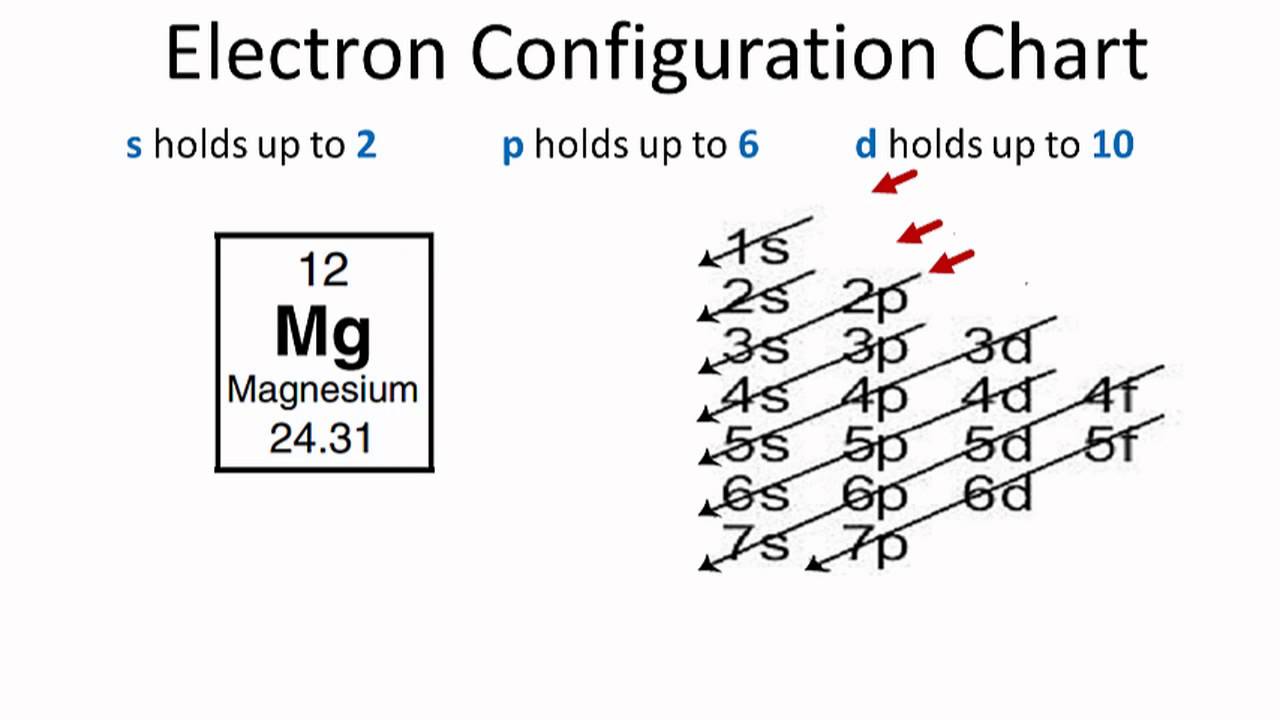
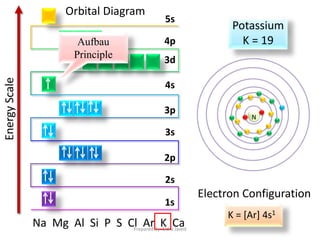
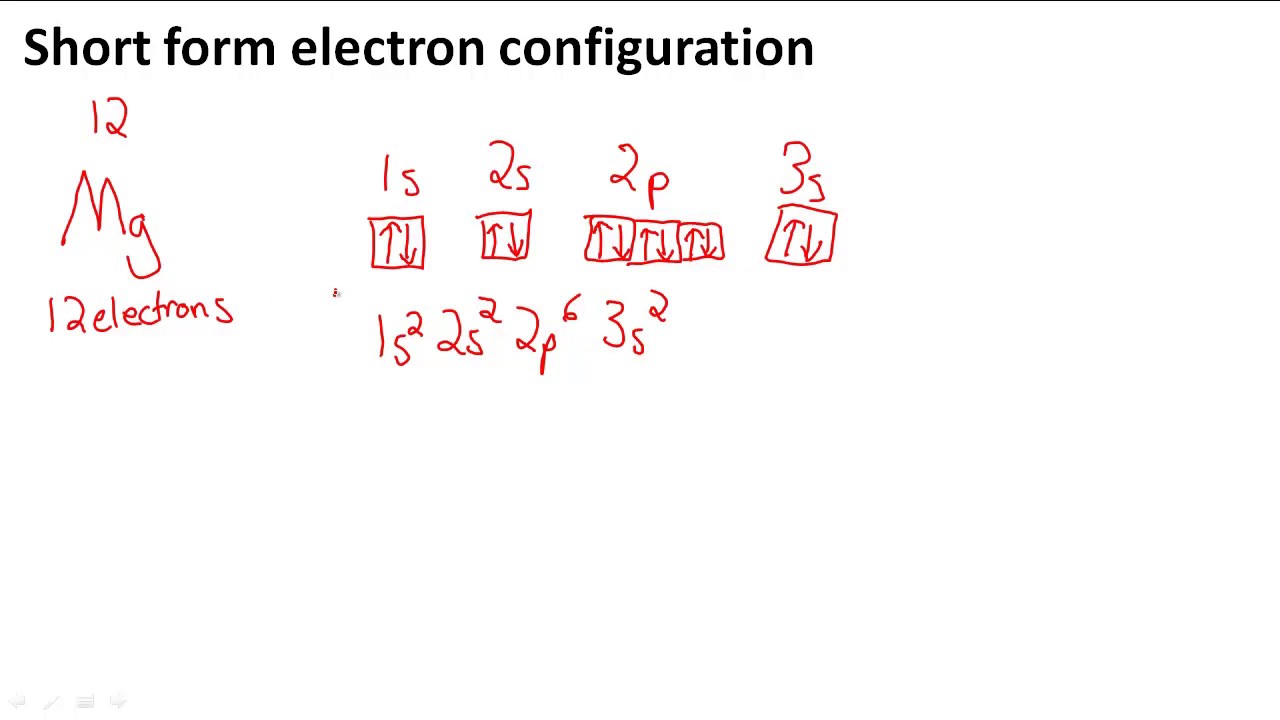
PDF Step by Step: Electron Configurations and Electron Orbital ... Step by Step: Electron Configurations and Electron Orbital Diagrams Electron Configurations Ex. 1) Mg: 1s 2 2s2 2p6 3s2 ↑↑↑ 1 = 1. st. layer (row #), s = orbital type , power of 2 = the 2 electrons in the 1s orbital **Move the Helium box next to Hydrogen (above Beryllium.) See the periodic table below. Mg 2+ Electron Configuration (Magnesium Ion) - YouTube In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We'll also look at why Magnesium forms a 2+ ion and how the electron con...
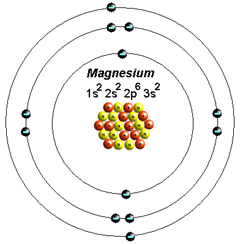
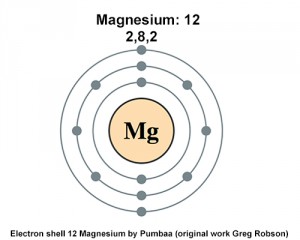
Electron Configuration for Magnesium (Mg) In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
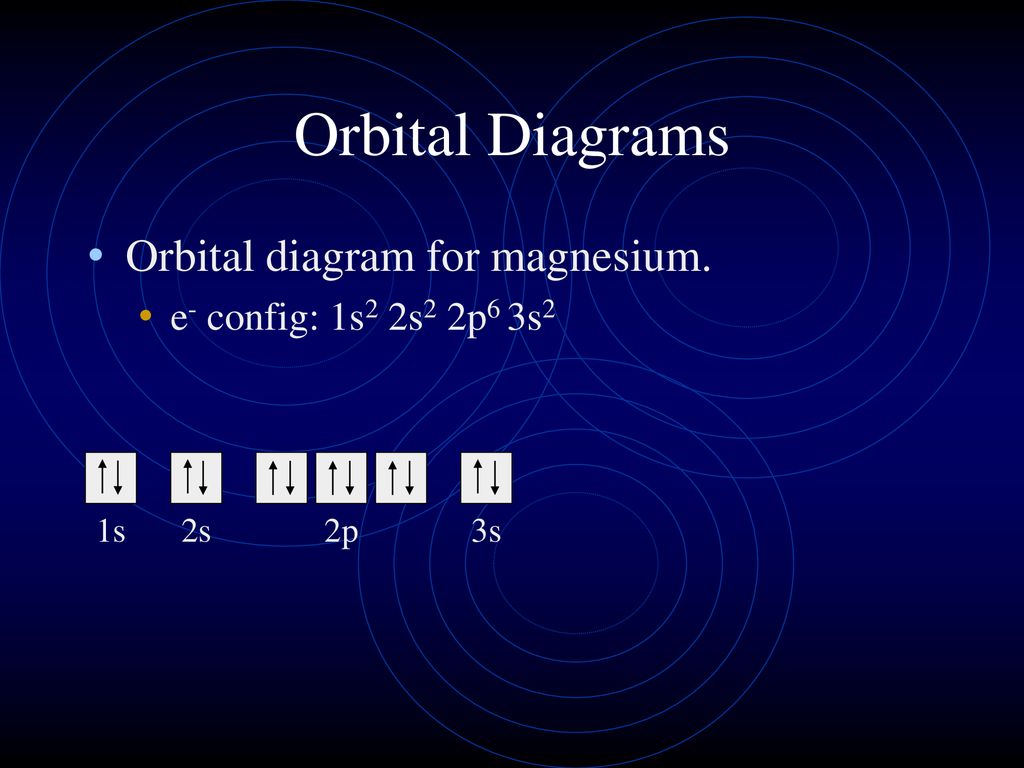
Orbital diagram for mg
Vanadium Orbital Diagram - Wiring Diagrams What element does the following orbital diagram represent? Mg (magnesium). What element does the the following orbital diagram represent? V (vanadium). Solutions for Chapter 8 Problem 4SAQ. Problem 4SAQ: Choose the correct orbital diagram for vanadium. step-by-step solutions; Solved by professors &. Oxidation States, +5,2,3,4. Answered: What is the orbital diagram for… | bartleby What is the orbital diagram for Magnesium Mg 12. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for Magnesium Mg 12. check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here. Molybdenum(Mo) electron configuration and orbital diagram Molybdenum (Mo) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
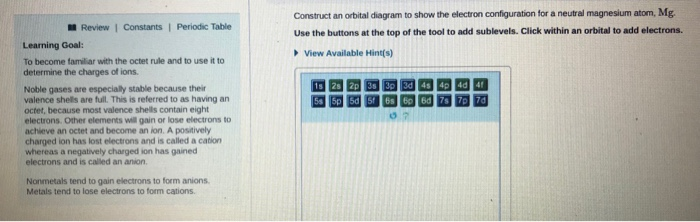
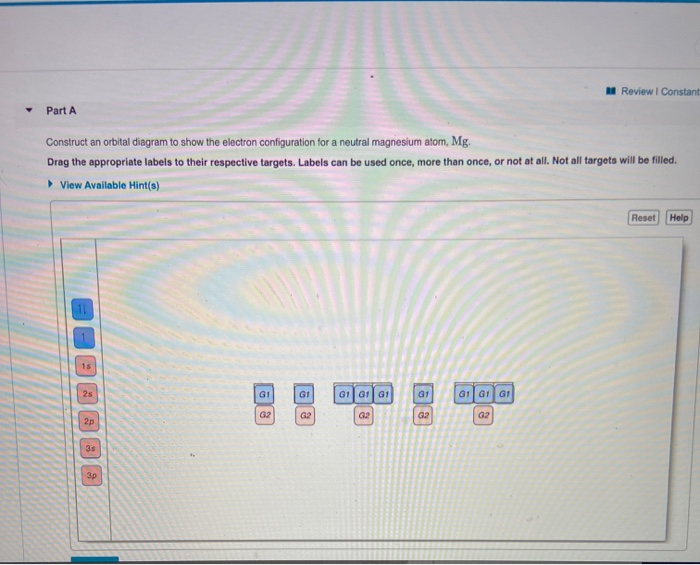
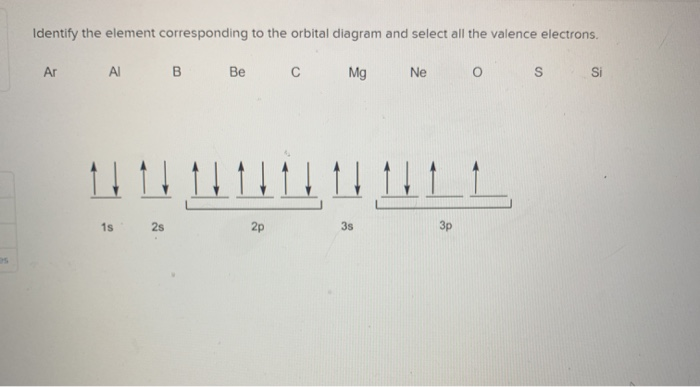
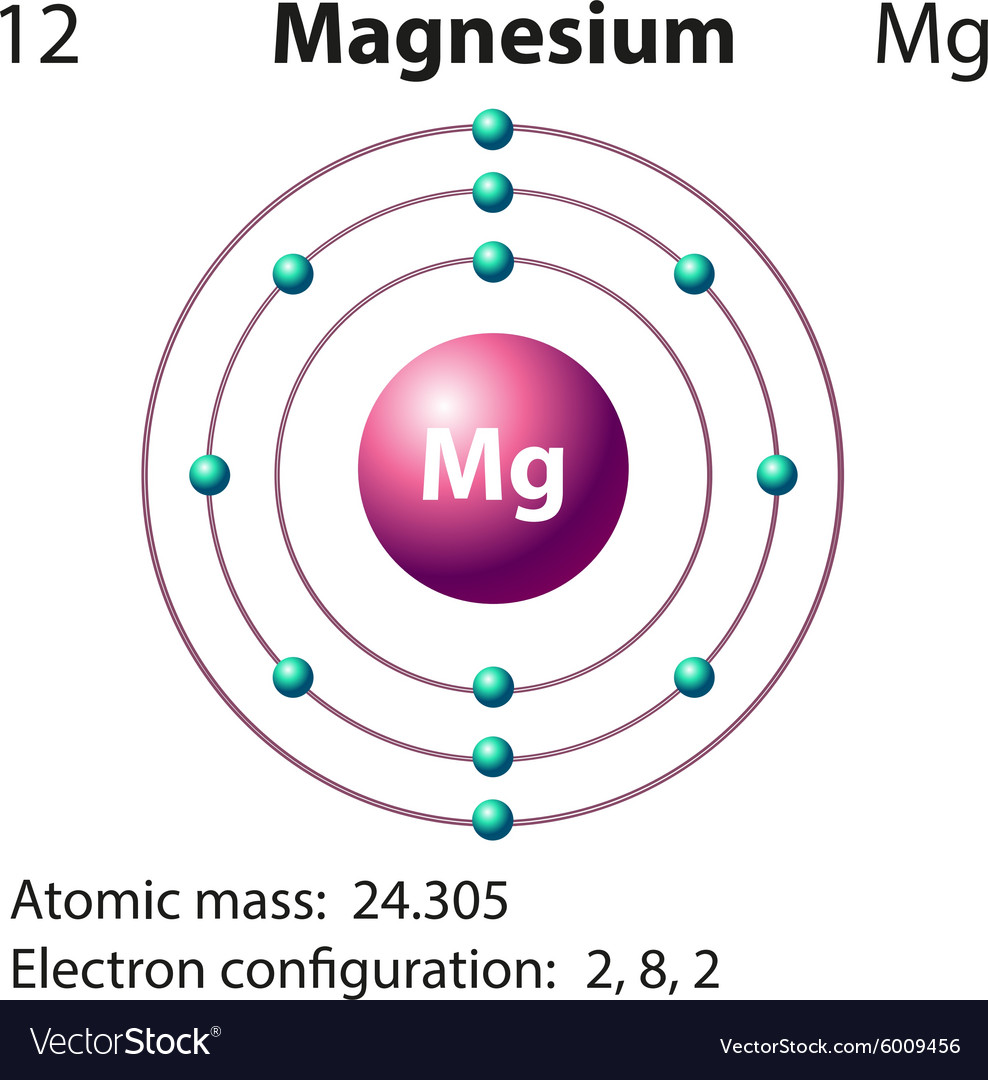
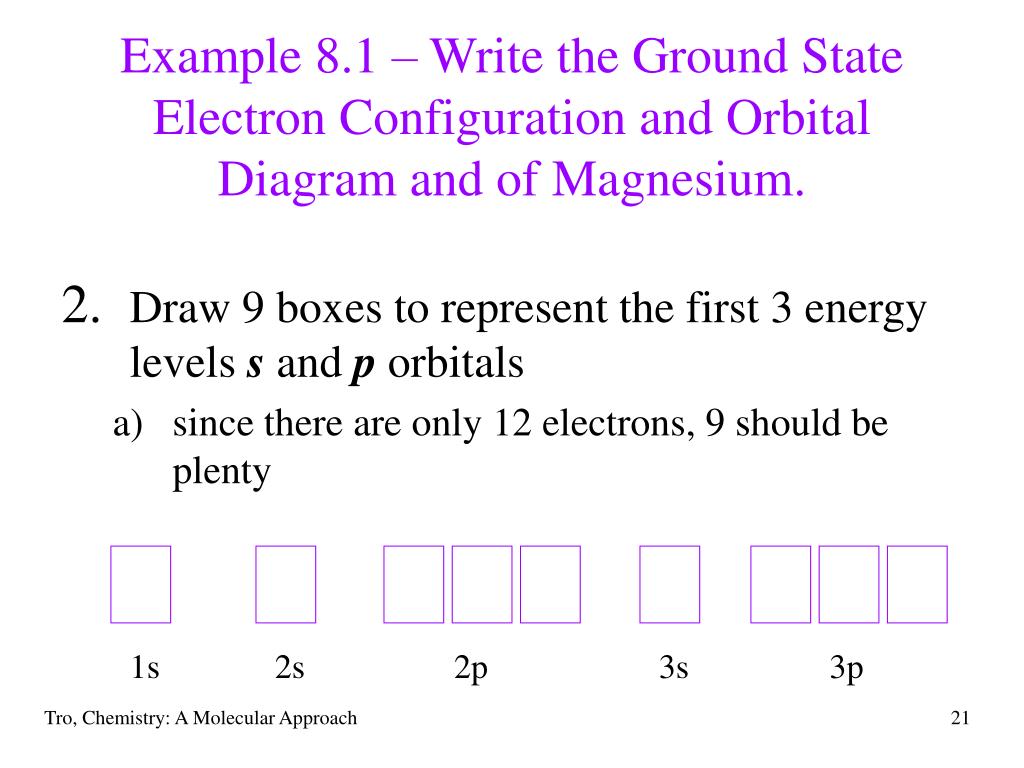
Orbital diagram for mg. Solved: Draw an orbital diagram for each element: (a ... a) The atomic number of magnesium is 12, and its electronic configuration is as follows, 1s 2 2s 2 2p 6 3s 2.Therefore, the orbital diagram for magnesium can be drawn as, Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ... Electronic Configuration of Magnesium Cation- Mg²+ - Bob ... Mg—> Mg²+ + 2e-Magnesium Cation Mg2+ Magnesium cation (Mg2+) is, as stated above, is a cationic /less stable derivative of Mg formed after It gives up two of its valency or outer electrons from its electron shell or orbital. Electronic Configuration of Mg2+ tells us how many electrons are arranged in each shell of the Mg2+ atom and its shape. Construct an orbital diagram to show the e... | Clutch Prep We're being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Mg is 12 and since it's a neutral element, this means Mg has 12 electrons.
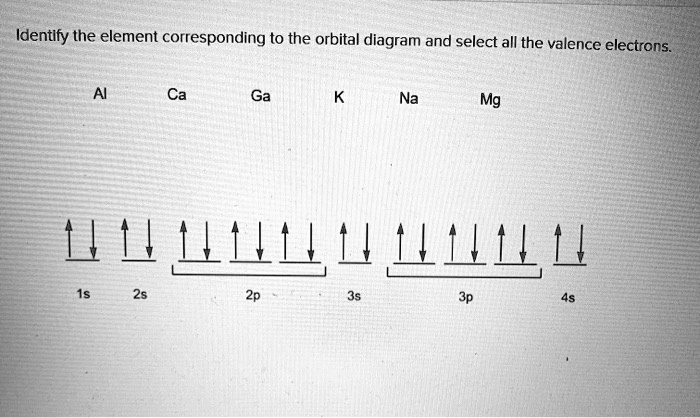
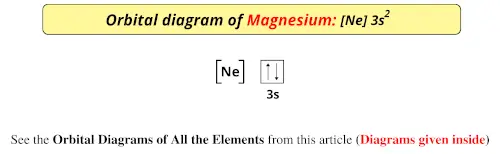
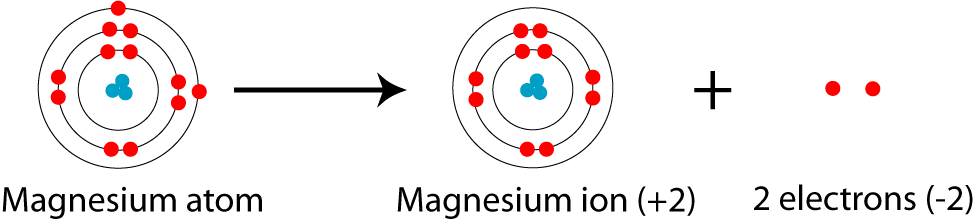
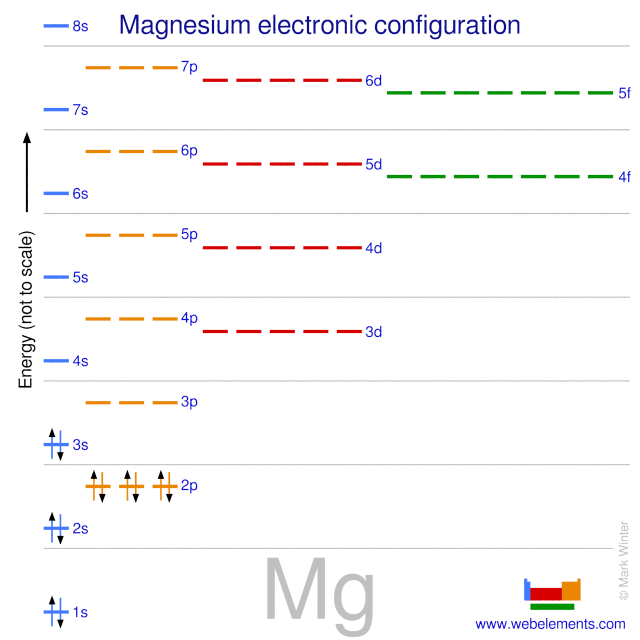
Orbital Diagram For Mg — UNTPIK APPS Orbital Diagram for Mg. quantum numbers and electron configurations there is only one orbital in the 2s subshell but there are three orbitals in the 2p subshell because there are three directions in which a p orbital can electron configurations how to write out the s p d f electron configurations for elements of atomic number z = 1 to 56 part 2 3 uses the rules on assigning electron ... What is the orbital diagram for magnesium? - Quora Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what... Magnesium(Mg) electron configuration and orbital diagram Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion (Mg 2+) Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency and valence electrons of magnesium are 2. Magnesium (Mg) Orbital diagram, Electron configuration ... The orbital diagram for Magnesium is drawn with 4 orbitals. The orbitals are 1s, 2s, 2p, and 3s. The Magnesium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, and the remaining two electrons in 3s orbital.
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... orbital notation for magnesium - Henchen Construction In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom (there are 12 electrons). The letter that designates the orbital type (the subshell, l), and; A superscript number that designates the number of electrons in that particular subshell. The notation describes the energy levels, orbitals and the number of electrons in each. the orbital ... what is the orbital diagram of the magnesium - Brainly.ph The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. laminiaduo7 and 5 more users found this answer helpful. heart outlined. Orbital Notation, Electron Config, Noble Gas Config ... Mg (Magnesium) - orbital diagram. S (Sulfur) - orbital diagram. O (Oxygen) - orbital diagram. N (Nitrogen) - orbital diagram. Si (Silicon) - orbital diagram. F (Fluorine) - orbital diagram. V (Vanadium) - orbital diagram. Hydrogen - electron configuration. Helium - electron configuration. Lithium - electron configuration. Beryllium - electron ...
Show the orbital diagram for the element Mg. | Study.com Show the orbital diagram for the element Mg. Orbital Diagram. The orbital Diagram is the most detailed form of the electron configuration for an element. The numbers relate to the energy level the ...
Molybdenum(Mo) electron configuration and orbital diagram Molybdenum (Mo) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Answered: What is the orbital diagram for… | bartleby What is the orbital diagram for Magnesium Mg 12. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for Magnesium Mg 12. check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here.
Vanadium Orbital Diagram - Wiring Diagrams What element does the following orbital diagram represent? Mg (magnesium). What element does the the following orbital diagram represent? V (vanadium). Solutions for Chapter 8 Problem 4SAQ. Problem 4SAQ: Choose the correct orbital diagram for vanadium. step-by-step solutions; Solved by professors &. Oxidation States, +5,2,3,4.
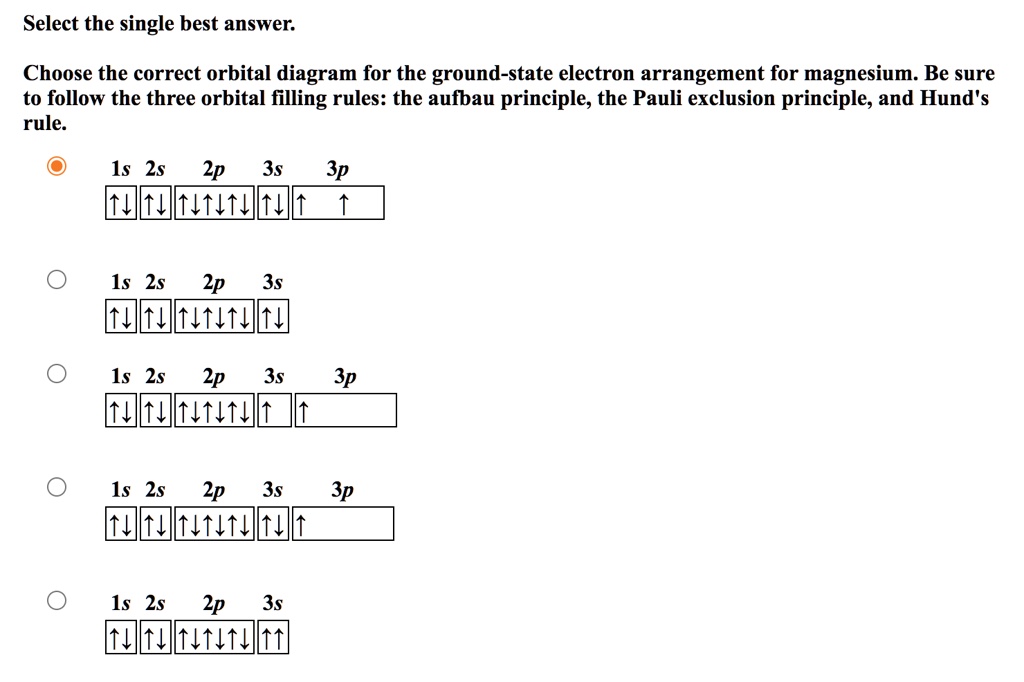


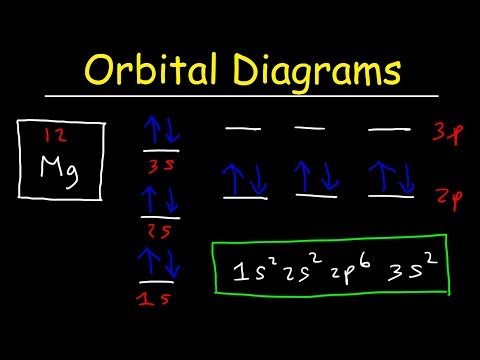
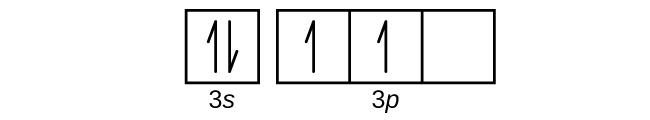





![6] (a) Write an orbital diagram for the ground state of ...](https://img.homeworklib.com/images/b7f46805-3b3a-4cf6-8603-c907004a6c53.png?x-oss-process=image/resize,w_560)













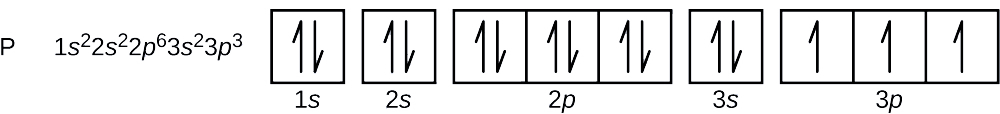
Comments
Post a Comment