42 lewis dot diagram n2
What is the Lewis structure of N2? | Socratic In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-. What is the Lewis structure for 2h2? - Book Vea A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion.
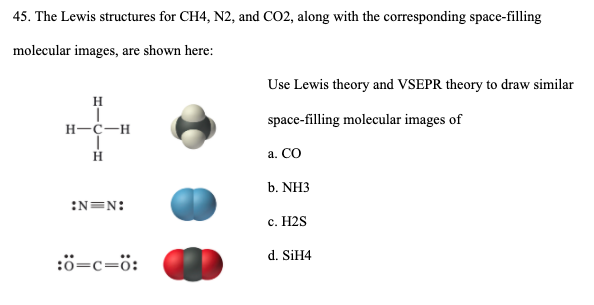
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.

Lewis dot diagram n2
What is the Lewis dot structure for N2? - Answers However, the carbonate anion, CO3^2- does have a Lewis dot structure. What is the Lewis dot structure for N2 look like? N(triple bond)N and then two dots on each N in which ever spot is open. Answered: lewis dot diagram | bartleby complete the lewis dot diagram for each of the following covalent compounds. ... N2(g)+3H2(g)=2NH3(g), a sample contains 4 N2 molecules and 14H2 molecules. When on... Draw electron dot structure of CO2,H2O,F2,N2 Click here👆to get an answer to your question ️ Draw electron dot structure of CO2,H2O,F2,N2. Solve Study Textbooks Guides. Join / Login >> Class 11 ... Draw electron dot structure of C O 2 ... Write the Lewis dot structure of C O molecule. Medium. View solution > Draw the electron dot structure of Propanone.
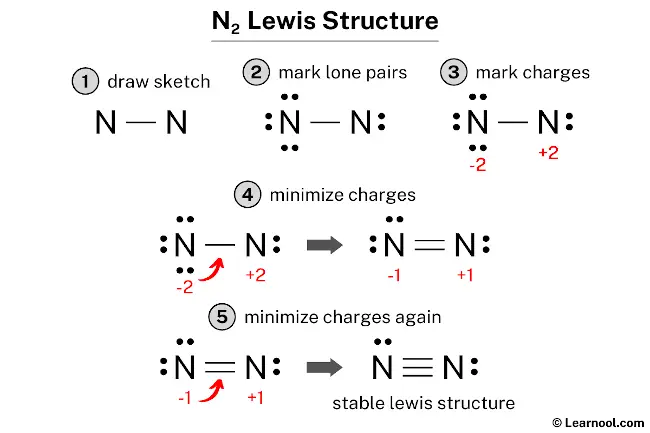
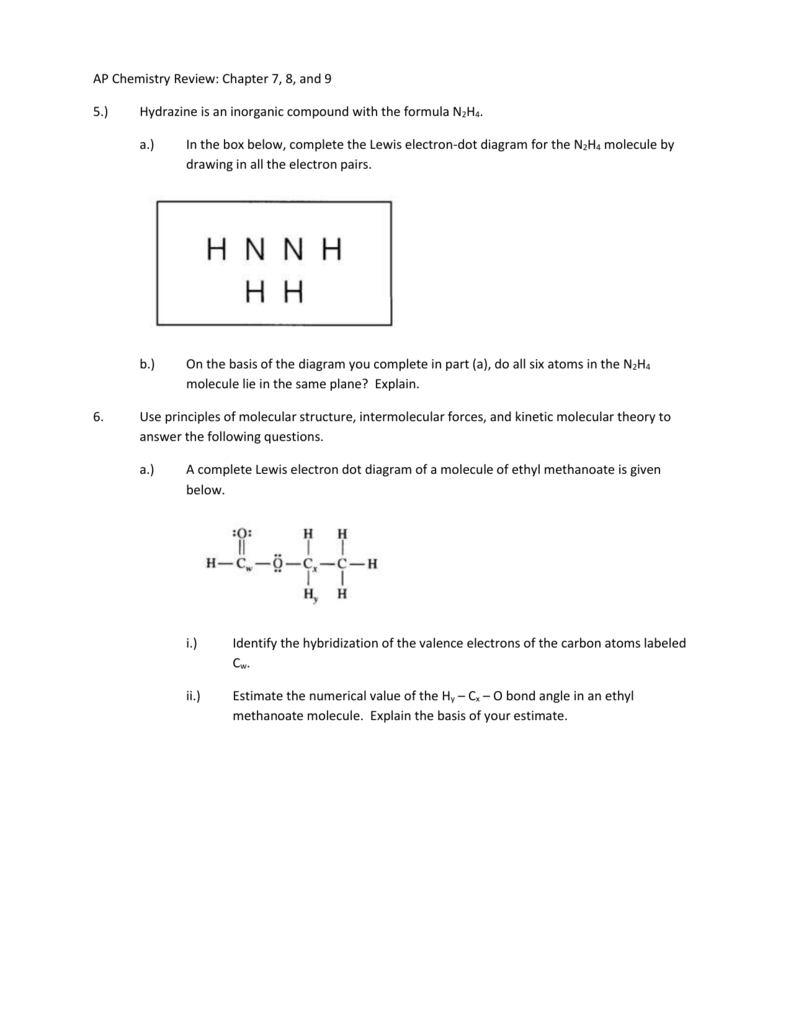
Lewis dot diagram n2. N2 Lewis Structure: Full Guide (2022 Updated) Steps In Drawing the N2 Lewis Structure To create a Lewis structure, determine first the number of valence electrons in each atom. Nitrogen has a total of ten valence electrons—five electrons on its outermost valence shell. After determining the total number of valence electrons., connect the atoms between electron pairs. Solved Answer the following questions about N2 and N2H4 ... N2 can react with H2 to form the compound N2H4. (f) The Lewis electron-dot diagram of N2H4 is shown below. (i) Based on the Lewis electron-dot diagrams of N2 and N2H4, compare the length of the nitrogen-to-nitrogen bond in N2 with the length of the nitrogen-to-nitrogen bond in N2H4. N2 Lewis Structure | Lewis Structure N2 | HND Assignment How is n2 formed in n2 lewis structure? The Lewis Structure or the Lewis Dot Structure or the Lewis Dot Diagram, named after Gilbert N. Lewis, shows the diagram of the atomic bonding of the molecules or an element. It shows the lone pairs of molecules existing in a molecule. Lewis Dot Diagram For N2 - schematron.org If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like? N (triple bond)N and then two dots on each N in which ever spot is open. Share to.Lewis structure - WikipediaWhat is the Lewis structure of N2
42 lewis dot diagram for n2 - Modern Wiring Diagram Lewis dot diagram for n2 Lewis Dot Diagrams of the Elements - STEM Sheets Lewis dot diagrams are a visual representation of the valence electrons on an atom of each individual element. These diagrams are used to draw Lewis dot structures, also know as electron dot structures or Lewis dot formulas, of compounds. Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. ⚗️Based on the Lewis electron-dot diagrams of n2 and n2h4 ... College. answer. answer. answered. Based on the Lewis electron-dot diagrams of n2 and n2h4, compare the length of the nitrogen-to-nitrogen bond in n2 with the length of the nitrogen-to-nitrogen bond in n2h4. 2. N2 Lewis Structure - Easy Hard Science The N 2 Lewis structure indicates that the N 2 molecule is perfectly symmetric. Therefore, N 2 is a nonpolar substance. Small nonpolar substances tend to be gasses. They tend to have low boiling points. For example, N 2 must be chilled to about -200 ℃ or -320 ℉ to liquify it.
N2 Lewis Structure, Molecular Geometry, and Hybridization ... Steps to Draw the Lewis structure of N2 Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen. Answered: Draw the Lewis Dot Structure for N2.… | bartleby Solution for Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or triple)? Is the bond polar or nonpolar and… Is N2 polar or nonpolar: Nitrogen polarity explained ... The Lewis dot structure for Nitrogen will be this: However, when two atoms of Nitrogen bind together, it has the following structure: Here both these atoms share six valence electrons to form a triple bond. These electrons are shared equally, and both Nitrogen atoms have one lone pair of electrons. N2 Polarity Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 1) Lewis dot structure of N2 is given below: (a) In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. (b) So N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. 2) Lewis dot structure of CCl4 is given below:
Nitrogen (N2) Molecule Lewis Structure Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's lewis structure.
PDF Lewis dot structure of n2 molecule Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
PPT PowerPoint Presentation - Chemical BONDING Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell!
N2 Lewis Structure: How to Draw the Dot Structure for N2 ... Drawing the Lewis Structure for N 2 Viewing Notes: Make sure you count the number of valence electrons correctly. Nitrogen is in group 5A (also called Group 15). Each Nitrogen atom has five valence electrons. Since there are two Nitrogen atoms in N 2 you have a total of ten valence electrons to work with.
Lewis Structure of N2 (Nitrogen Gas) - YouTube How to Draw the Lewis Structure of N2 - with explanation!Check me out:
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
Lewis Structure For Nitrogen Gas N2 - Novocom.top lewis n2 dot diagram chemical bonding structure nitrogen gas chemistry bond o2 oxygen bonds structures visionlearning double between atoms library . nitrogen structure gas molecule molecular diagram electron atoms electrons tea why air does zorach alex which .
Draw the Lewis structure for N2. Nitrogen is an unreactive ... The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
N2 Lewis Structure| Hybridization & Molecular Geometry ... N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. 6. Explain o2 lewis structure in simplest form. Two oxygen atoms are joined by a ...
Draw Lewis dot structures of the diatomic molecules 02 and ... Oxygen gas has the molecular formula of O2 and nitrogen gas is N2. The Lewis dot structures that represent both diatomic gases are::O=O: :N:::N:
PDF Lewis Dot Structures Pogil Key - Hudson City School District 9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
Draw electron dot structure of CO2,H2O,F2,N2 Click here👆to get an answer to your question ️ Draw electron dot structure of CO2,H2O,F2,N2. Solve Study Textbooks Guides. Join / Login >> Class 11 ... Draw electron dot structure of C O 2 ... Write the Lewis dot structure of C O molecule. Medium. View solution > Draw the electron dot structure of Propanone.
Answered: lewis dot diagram | bartleby complete the lewis dot diagram for each of the following covalent compounds. ... N2(g)+3H2(g)=2NH3(g), a sample contains 4 N2 molecules and 14H2 molecules. When on...
What is the Lewis dot structure for N2? - Answers However, the carbonate anion, CO3^2- does have a Lewis dot structure. What is the Lewis dot structure for N2 look like? N(triple bond)N and then two dots on each N in which ever spot is open.



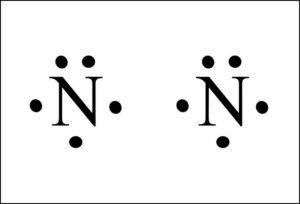













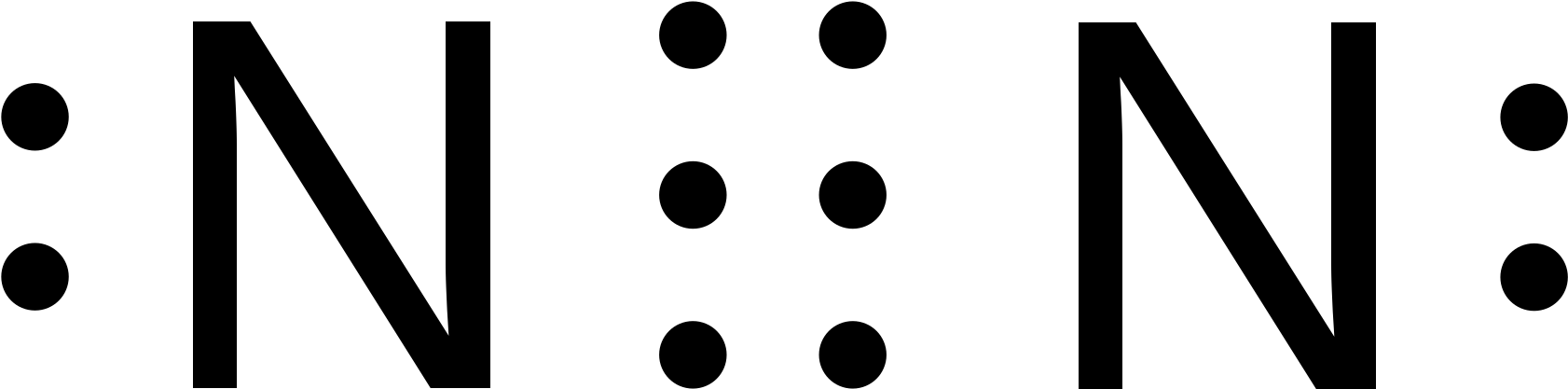









Comments
Post a Comment