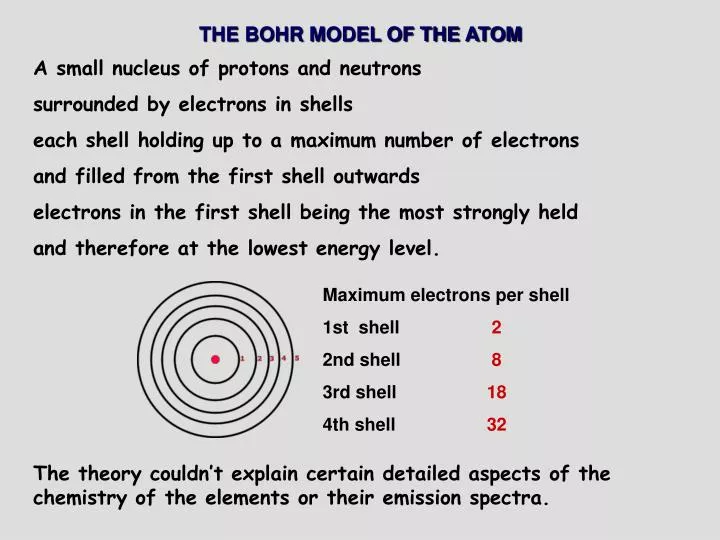
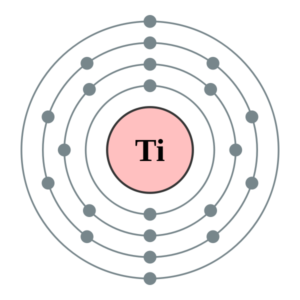
41 titanium bohr diagram
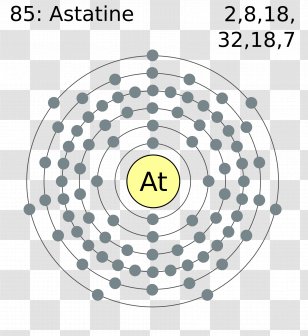
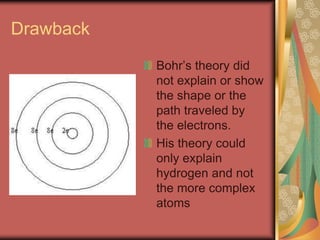
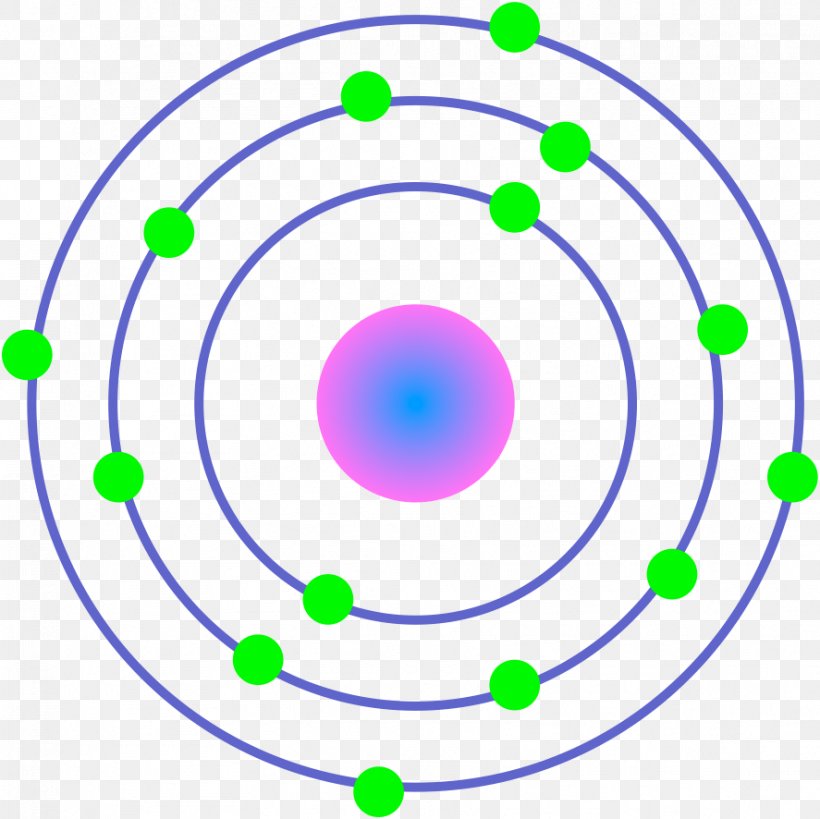
Atom Diagrams: Electron Configurations of the Elements 05/11/2019 · It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. For that, we have electron shell diagrams.. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.. For each electron shell atom diagram, the element symbol is listed in the nucleus. Titanium, atomic structure - Stock Image - C018/3703 ... Titanium (Ti). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of titanium-48 (atomic number: 22), the most common isotope of this element. The nucleus consists of 22 protons (red) and 26 neutrons (orange). 22 electrons (white) successively occupy available electron shells (rings).
Assisting students with assignments online - Success Essays Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Titanium bohr diagram
Titanium (Ti) - Chemical Elements.com Name: Titanium Symbol: Ti Atomic Number: 22 Atomic Mass: 47.867 amu Melting Point: 1660.0 °C (1933.15 K, 3020.0 °F) Boiling Point: 3287.0 °C (3560.15 K, 5948.6 °F) Number of Protons/Electrons: 22 Number of Neutrons: 26 Classification: Transition Metal Crystal Structure: Hexagonal Density @ 293 K: 4.54 g/cm 3 Color: silverish Atomic Structure sciencing.com › calculate-valency-2790How to Calculate Valency - Sciencing Feb 10, 2020 · Valency is a measure of the ability of an atom to bond with other atoms. The higher the number of valent electrons, the more reactive the atom or molecule is. Electrons will occupy the most stable position first. The inner orbital holds up to 2 electrons. The next orbital holds up to 8 electrons. Location - Titanium Family: Transition Metals Group: 4 Period: 4 OTHER ELEMENTS IN THE SAME FAMILY/GROUP Zirconium Hafnium Rutherfordium BOHR DIAGRAM
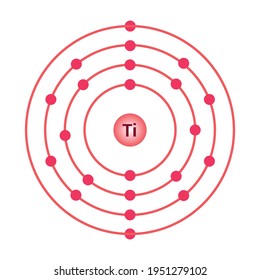
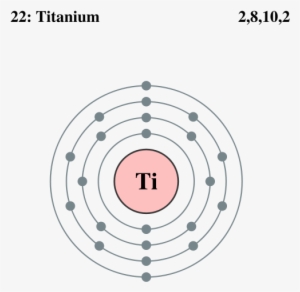
Titanium bohr diagram. 40 choose the correct orbital diagram for vanadium ... how many valence electrons does vanadium have The free energy of an electronâ hole pair is smaller than the band gap energy due to the translational entropy of the electrons and holes â ¦ The chemical symbol for Vanadium is V. Vanadium is a hard, silvery grey, ductile, and malleable transition metal. 1: Bohr diagrams: Bohr diagrams indicate how many electrons fill each principal shell. Bohr Model of all Elements (Diagrams + Chart Inside) Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Titanium Bohr Diagram - schematron.org This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons. The first shell has two electrons, the second shell has eight electrons, the third shell also has eight electrons, and the fourth and last shell has 4 electrons which makes a . › valence-electronsValence Electrons - GeeksforGeeks Mar 02, 2022 · An electron dot diagram is a representation of an atom’s valence electrons that employs dots to surround the element’s symbol. The number of dots corresponds to the atom’s valence electrons. With no more than two dots on each side, these dots are positioned to the right and left, above and below the symbol.
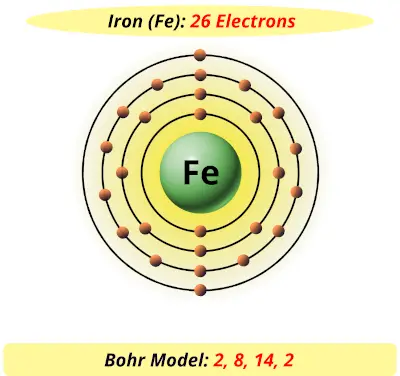
Atoms, Elements, & the Periodic Table Jeopardy Template This type of charge is associated with a proton., This subatomic particle is not found inside of the nucleus., Titanium has an atomic number of 22 and a mass of 48. How many protons and electrons does an atom of titanium have?, An atom that has gained 2 electrons can be described as this (state name and charge). Sodium Bohr Model — Diagram, Steps To Draw - Techiescientist Sodium Bohr Model — Diagram, Steps To Draw. Sodium is a highly reactive metal element. It has the atomic number 11 and is represented by the symbol Na. It belongs to group 1A of the periodic table and hence, is an alkali metal. It is silvery-white in appearance and exists in nature in the form of minerals such as sodalite, rock salt, feldspar ... Titanium Bohr Diagram This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons. The first shell has two electrons, the second shell has eight electrons, the third shell also has eight electrons, and the fourth and last shell has 4 electrons which makes a . cep-arch.nl Před 1 dnem · email protected] [email protected] 25 3. Metals do not form bonds with other metals How is this different from the ionic bonds formed in the previous part of the activity?
blog.prepscholar.com › atomic-radius-trendUnderstanding Atomic Radius Trends: The 2 Key Principles Feb 07, 2021 · Below is a very simplified diagram of four atoms, all about the same size. The top two atoms are connected by a covalent bond, which causes some overlap between the atoms. The bottom two atoms are noble gas atoms, and they are connected by Van der Waals forces that don't allow the atoms to get as close together. Sulfur(S) electron configuration and orbital diagram Sulfur (S) excited state electron configuration and orbital diagram. This electron configuration shows that the last shell of the sulfur atom has six unpaired electrons (3s 1 3p x1 3p y1 3p z1 3d xy1 3d yz1 ). So the valency of sulfur is 6. From the above information, we can say that sulfur exhibits variable valency. Bohr Diagram 3AC Bohr Diagram | 101 Diagrams. Read more... Comments . The Lab Lads: Bohr Models! Diagram Of An Atom Of Lithium - Aflam-Neeeak. How To Draw A Bohr Model Of An Atom - slideshare. Atoms Through Time timeline | Timetoast timelines. Bohr model - Wikiquote. phosphorus. BOHR DIAGRAM - Unmasa Dalha. Bohr Diagram of Titanium - Weebly This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons. The first shell has two electrons, the second shell has eight electrons, the third shell also has eight electrons, and the fourth and last shell has 4 electrons which makes a total of 22 electrons in one titanium atom.
Superconductivity - Wikipedia Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor.Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered even down to near absolute zero, …
(PDF) Callister Materials science and Engineer | KTK ... attached are materials science engineering by william d callister and my paper work for bionergy renewable energy. thanks
Titanium (Ti) - Periodic Table (Element Information & More) Titanium is a metal having a silvery white metallic color. Density of titanium is 4.507 g/cm 3 which is 60% denser than aluminum. The melting point of titanium is 1668 °C and its boiling point is 3287 °C. The crystal structure of titanium is HCP (Hexagonal close packing).
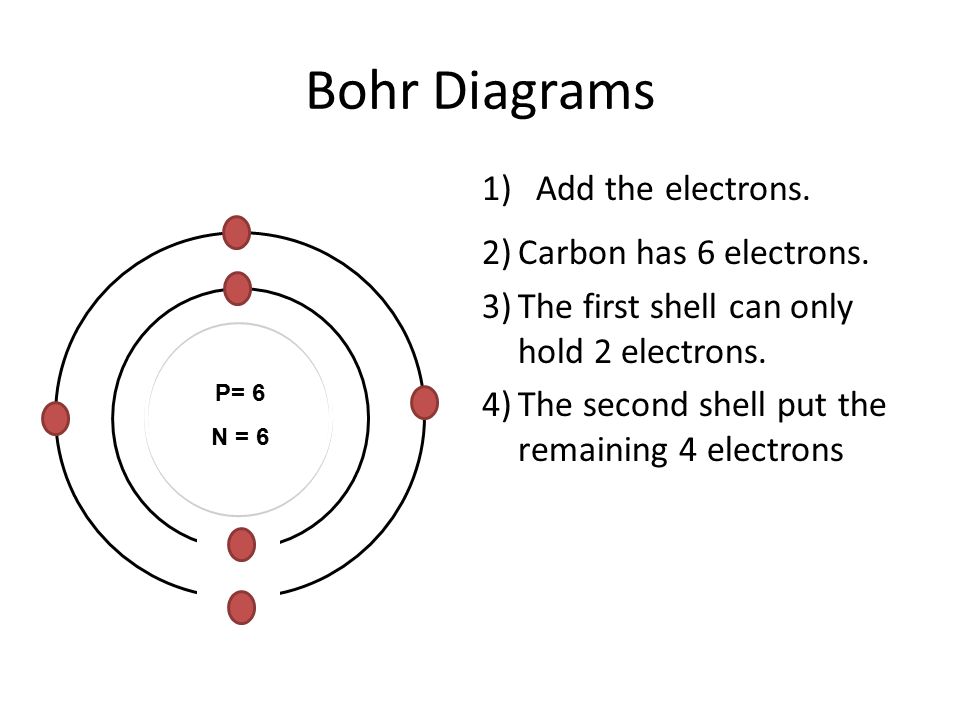
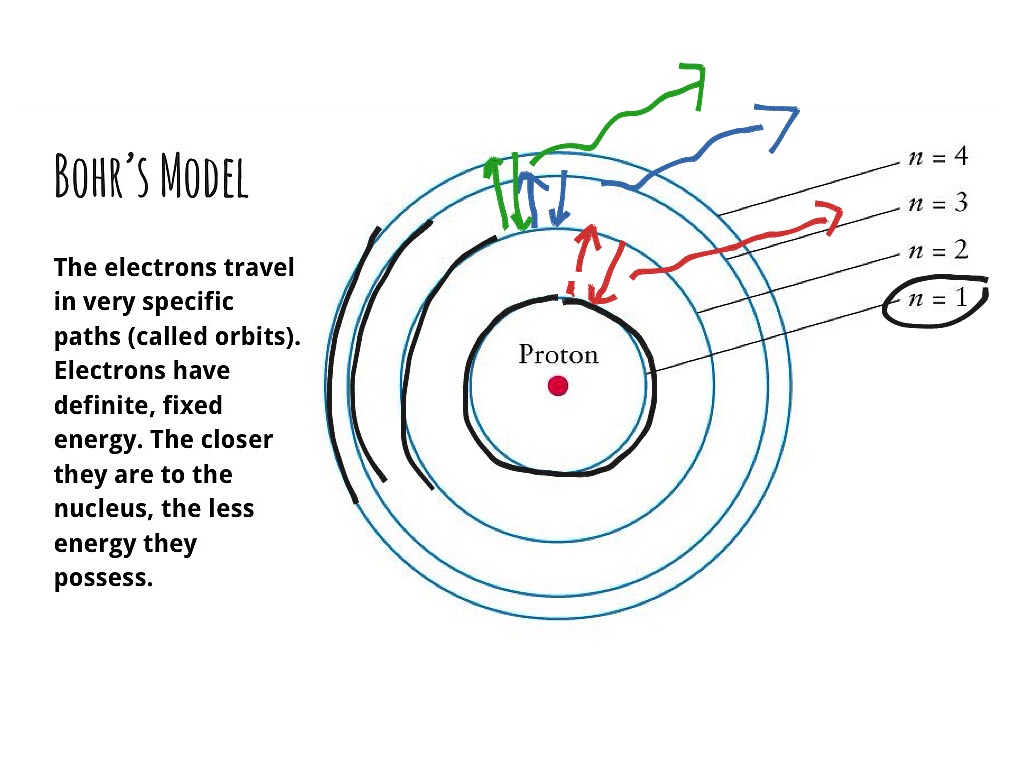
PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
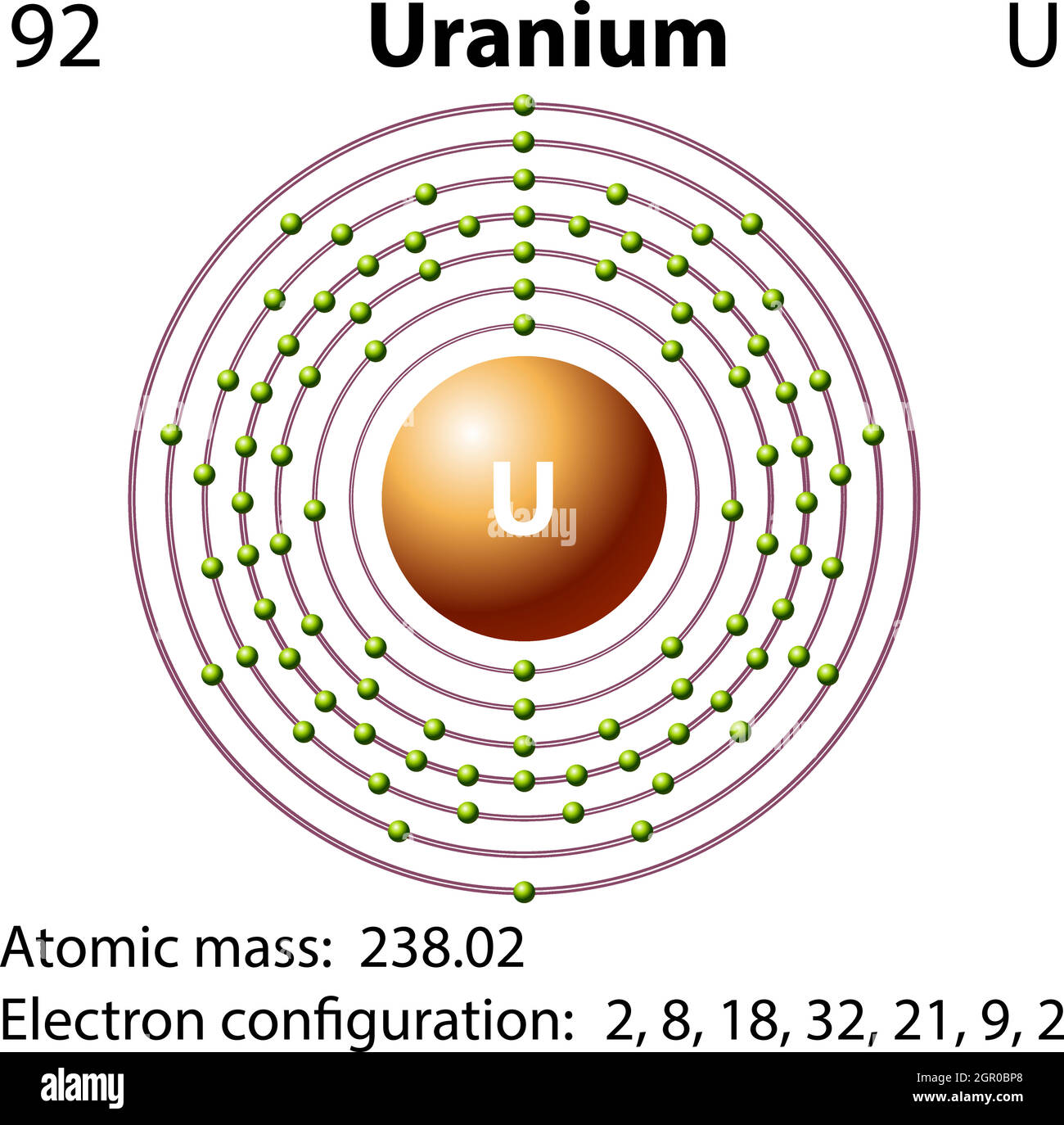
periodictableguide.com › atomic-mass-of-all-elementsAtomic Mass of all Elements (Chart + Rounded Values Inside) Mar 07, 2021 · Note: The Atomic masses are represented in the Atomic mass unit (u). The elements whose atomic masses are written in bracket ( ) are the synthetic elements and their atomic masses values represent the Atomic Mass of the most stable isotope.
How to Draw Bohr-Rutherford Diagrams - Potassium - YouTube How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
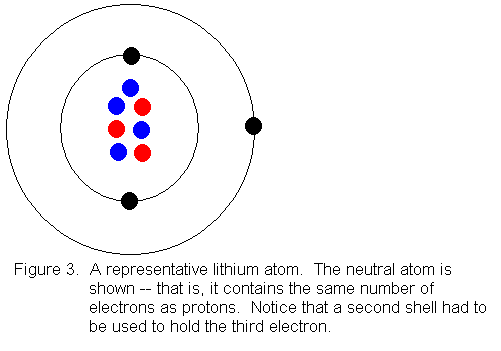
Lithium Bohr Model — Diagram, Steps To Draw - Techiescientist Hence, the final Bohr model of the Lithium atom consists of 3 protons and 4 neutrons inside the nucleus, and 3 electrons revolving around the nucleus. There are 2 electrons present in the K shell and 1 electron in the L shell. Deriving Lewis Diagram from Bohr Model. The Lewis diagram of an atom represents its atomic structure in the pictorial form.
Cambridge IGCSE Chemistry Coursebook (fourth ... - Issuu 09/06/2014 · Complete the diagram below to show how the atoms of argon are arranged at –188 °C. represents one atom of argon [2] [Cambridge IGCSE® Chemistry 0620/21, Question 3, November 2010] Original ...
Atoms, Elements, & the Periodic Table This type of charge is associated with a proton., This subatomic particle is not found inside of the nucleus., Titanium has an atomic number of 22 and a mass of 48. How many protons and electrons does an atom of titanium have?, An atom that has gained 2 electrons can be described as this (state name and charge).
Basic Info Atomic Symbol : Ti Atomic Number: 22 Atomic Mass: 47.867 Group: Transition Metals Year of Discovery: 1791 Discoverer: Reverend William Gregor Melting Point: 1668 degrees Celsius Boiling Point: 3287...
Titanium(Ti) electron configuration and orbital diagram Titanium (Ti) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Bohr-Rutherford Diagram of Zinc (Zn) - YouTube Zinc is the first element for which the third shell is truly full. Its electron arrangement is 2-8-18-2. That means there are EIGHTEEN electrons on the third...
PDF Bohr Diagram For Fr - 178.128.217.59 difference between the bohr rutherford diagram, niels bohr facts nobelprize org, how to draw bohr diagrams slideshare, bohr diagram of titanium weebly, category bohr model wikimedia commons, atomic structure the bohr model dummies, how to bohr diagram slideshare, how to make a 3 d bohr model sciencing, bohr diagrams and lewis dot review ...
PDF Periodic Table, Drawing Bohr Diagrams Periodic Table, Drawing Bohr Diagrams. Periodic Table •The Periodic Table provides information on the physical and chemical properties of elements ... Titanium name 47.9 atomic mass ion charge(s) lgp 20 n le 19p 20 n o first shell up to 2 electrons second shell up to 8 electrons nucl
titanium - Compounds | Britannica titanium - titanium - Compounds: In its compounds, titanium exhibits oxidation states of +2, +3, and +4, as in the oxygen compounds titanium monoxide, TiO, dititanium trioxide, Ti2O3, and titanium dioxide, TiO2, respectively. The +4 oxidation state is the most stable. The chemistry of titanium in the +2 state is rather restricted. By contrast, many compounds are formed by titanium in the +3 state.
Bohr Diagram for Arsenic, Faraday Law Electrolysis BOHR DIAGRAM FOR ARSENIC INFO: Titanium is a chemical element with the symbol Ti and atomic number 22. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. You can find metric conversion tables for SI units, as well as English units, currency, and other data.
Bohr Rutherford Diagram For The First 20 Elements Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ...
Calcium Bohr Diagram - schematron.org Powered by Create your own unique website with.• bohr rutherford diagram for calcium • bohr rutherford diagram • bohr rutherford diagram for carbon • bohr rutherford diagram of beryllium ok, i need to draw a diagram of a Titanium atom. but it has to be a bohr-rutherford diagram. Titanium is number 22 on the periodic table if im correct. i .
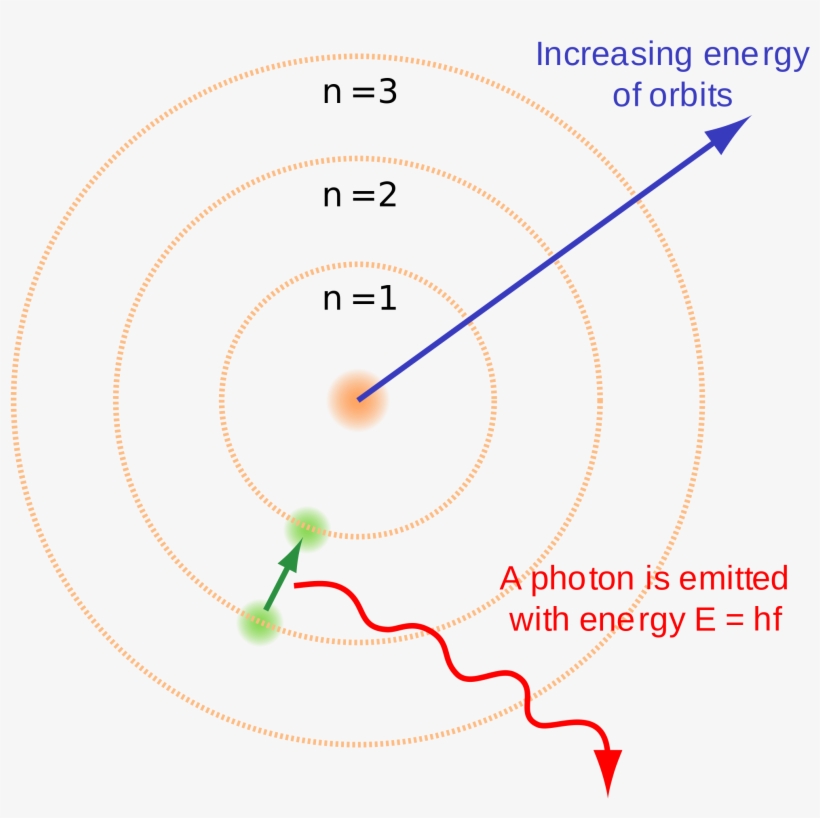
Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
Assignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Elodea Leaf Cell Diagram Elodea Leaf Cell Diagram. The Elodea leaf is composed of two layers of cells. Only one layer of cells is in focus when using the high. Examining elodea (pondweed) under a compound microscope. solution) and a coverslip and observe the chloroplasts (green structures) and the cell walls. In this lesson, students microscopically observe various ...
Location - Titanium Family: Transition Metals Group: 4 Period: 4 OTHER ELEMENTS IN THE SAME FAMILY/GROUP Zirconium Hafnium Rutherfordium BOHR DIAGRAM
sciencing.com › calculate-valency-2790How to Calculate Valency - Sciencing Feb 10, 2020 · Valency is a measure of the ability of an atom to bond with other atoms. The higher the number of valent electrons, the more reactive the atom or molecule is. Electrons will occupy the most stable position first. The inner orbital holds up to 2 electrons. The next orbital holds up to 8 electrons.
Titanium (Ti) - Chemical Elements.com Name: Titanium Symbol: Ti Atomic Number: 22 Atomic Mass: 47.867 amu Melting Point: 1660.0 °C (1933.15 K, 3020.0 °F) Boiling Point: 3287.0 °C (3560.15 K, 5948.6 °F) Number of Protons/Electrons: 22 Number of Neutrons: 26 Classification: Transition Metal Crystal Structure: Hexagonal Density @ 293 K: 4.54 g/cm 3 Color: silverish Atomic Structure















:max_bytes(150000):strip_icc()/Titanium-58b602395f9b5860464c4d8e.jpg)

Comments
Post a Comment