40 lewis dot diagram of co2
chemhelps.com › co2-lewis-structureCO2 Lewis Structure – Lewis Dot Structure | Chem Helps CO2 Lewis Dot Structure. When drawing the Lewis structure of the carbon dioxide molecule, the carbon and an unpaired electron of oxygen share with each other. As a result, a single covalent bond between carbon and oxygen occurs. However, in this case, carbon and oxygen cannot complete the octet. For carbon and oxygen to complete its octet, other unpaired electrons must also share. Chemistry Worksheet Lewis Dot Structures Ionic Compounds ... The lewis dot diagram for carbon dioxide also shows that two pairs of electrons are shared. Justify your answer using lewis electron dot structures. In section 4 7 we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anions.
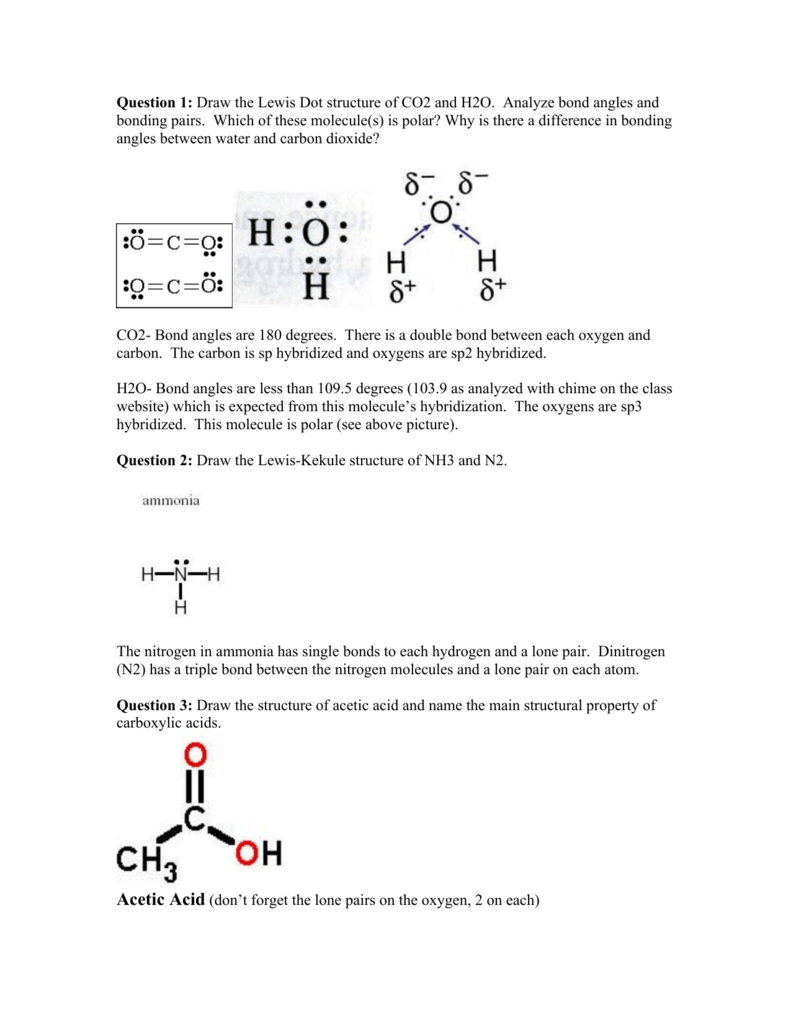
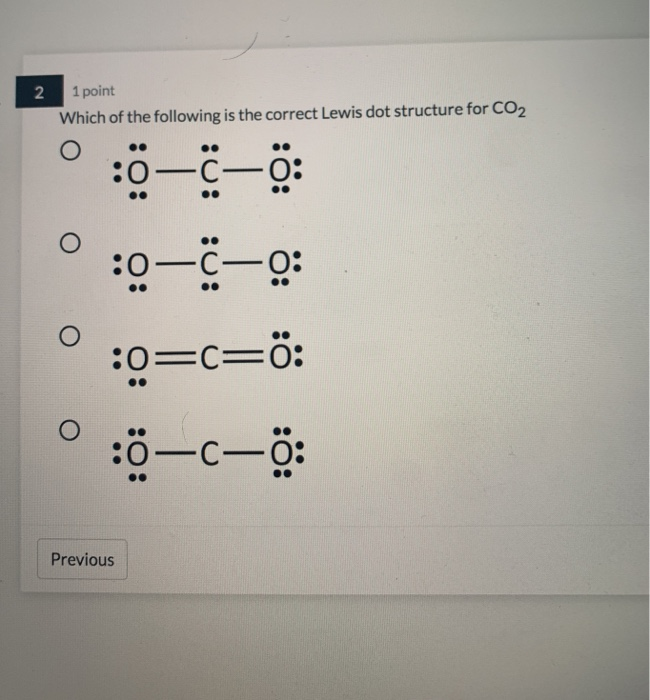
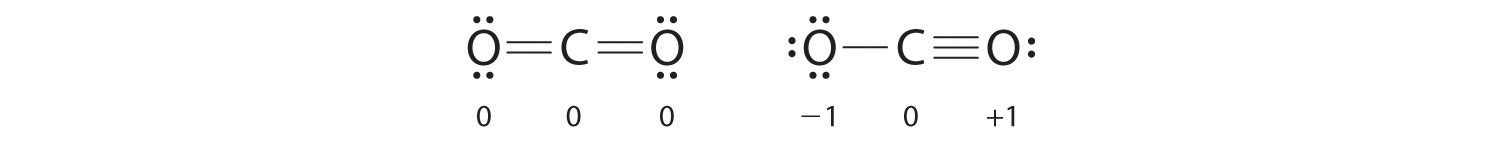
What is wrong with this Lewis dot structure for the CO2 ... What is wrong with this Lewis dot structure for the CO2 molecule? O It should have the single bond replaced with a double bond, and two lone pairs on the oxygen atom at that end. O It should have the triple bond replaced with a double bond, and two lone pairs on the oxygen atom at that end.

Lewis dot diagram of co2
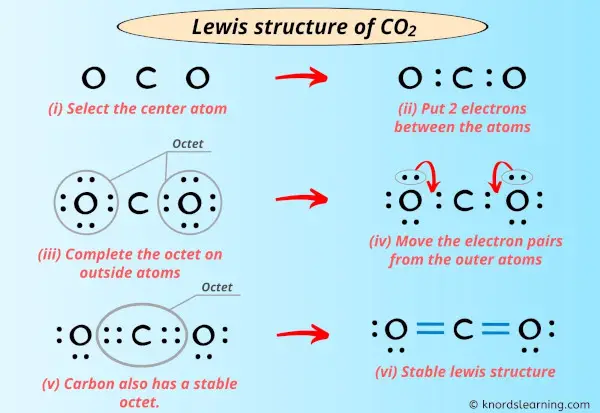
what is the shape of carbon dioxide - Lisbdnet.com What is the dot structure of carbon dioxide? CO2 Lewis Structure Setup. Carbon has four valence electrons that form a total of four bonds. So carbon is shown with four dots around it. Oxygen needs just two bonds, represented as the lone dots to the left and right of the O atoms. The pairs of dots above and below the O's won't bond. wellcometreeoflife.org › co2-lewis-structureCO2 Lewis Structure (2021 UPDATED) All You Need To Know Dec 22, 2021 · The number of valence electrons of a carbon atom is four which forms four bonds. The CO2 Lewis Structure is where the central carbon atom is the central atom, the least electronegative atom with O surrounded by four dots. The total valence electrons, including valence shells, are eight electrons pairs with no lone pair. › how-to-draw-lewisHow To Draw Lewis Structure For Co2? | iLoveMyCarbonDioxide Nov 25, 2021 · What Is The Lewis Diagram For Co2? The Lewis structure of carbon dioxide (CO) consists of two oxygen atoms and one carbon atom. The carbon atom in the CO has two double bonds. The valence shells of carbon atoms and oxygen atoms each contain two lone pairs. Linearity is the characteristic of CO.
Lewis dot diagram of co2. Chemistry Worksheet Lewis Dot Structures Ionic Compounds ... The lewis dot diagram for carbon dioxide also shows that two pairs of electrons are shared. Lewis dot structure mega worksheet answer key lewis structure worksheet 1 miss shafers lewis dot structure drill sheet principles of inorganic chemistry answers here are two. Lewis Dot Structure | Chem Helps - ChemHelps can help you ... A Lewis Structure or Lewis Dot Structure is a formula developed to concretely express the bond formation between chemical species (atom, molecule, ion). Lewis Structure shows the electrons in the last layer of the atom with dots around the symbol of the atom. How Many Valence Electrons Does Co2 Have, Carbon Dioxide Lewis dot framework is a pictorial representation the the setup of the valence shell electrons in the molecule. These valence electron are stood for by drawing dots roughly the individual atoms, thus the Lewis period structure. Drawing lines stand for the bonds developed in the molecule. Lewis Electron Dot Structures: Steps, Examples and Limitations Lewis Electron Dot Structure An atom is represented by its chemical symbol. Lines representing bonds between atoms are drawn. Lone pairs are drawn as dots. Instead of a line to represent a bond a pair of dots (2 electrons) can also be used. By placing dots or crosses around the chemical symbol of the atom valence shell electrons are represented.
Lewis Structures: Dot Symbols, Diagrams, Examples, Questions The Lewis structure for oxygen molecule is as shown below- Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Lewis Structure Worksheet Answer Key - Worksheet Live Lewis electron dot structure with answer key displaying top 8 worksheets found for this concept. Lewis structure worksheet answer key. H 2 o m. N 2 o i. For those of you that enjoy such things some more lewis structures to draw. Cf 2 h 2 e. Preferred customer created date. N3 Lewis Dot Structure - aunitedkingdomfilm.com Review what a Lewis dot diagram is and. Follow these simple steps to draw Lewis dot structures. A Lewis dot structure for SeO3 is drawn with an Se in the center with two lines connecting it to two Os and one double line connecting it to an O. Lewis Structure of CO2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the ... Lewis Dot Structure For Hcho Lewis dot structures step 1: The first step in drawing the lewis dot structure for ethane (c_2h_6) is to determine how many valence electrons are available for the molecule. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions.
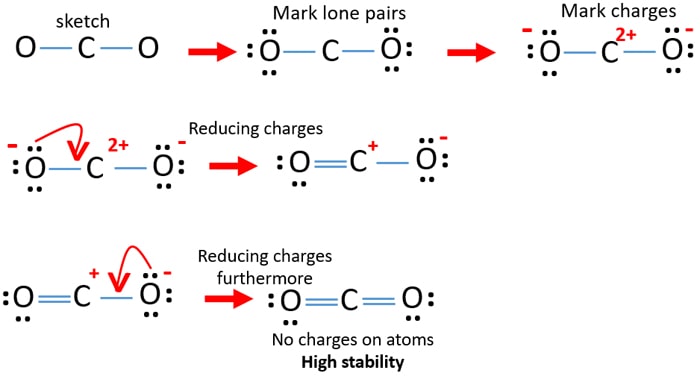
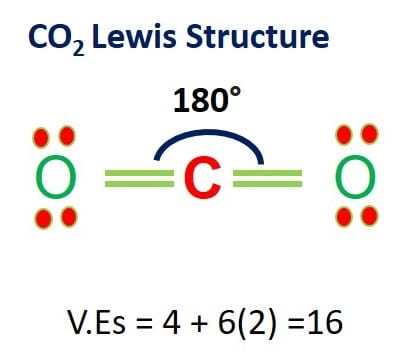
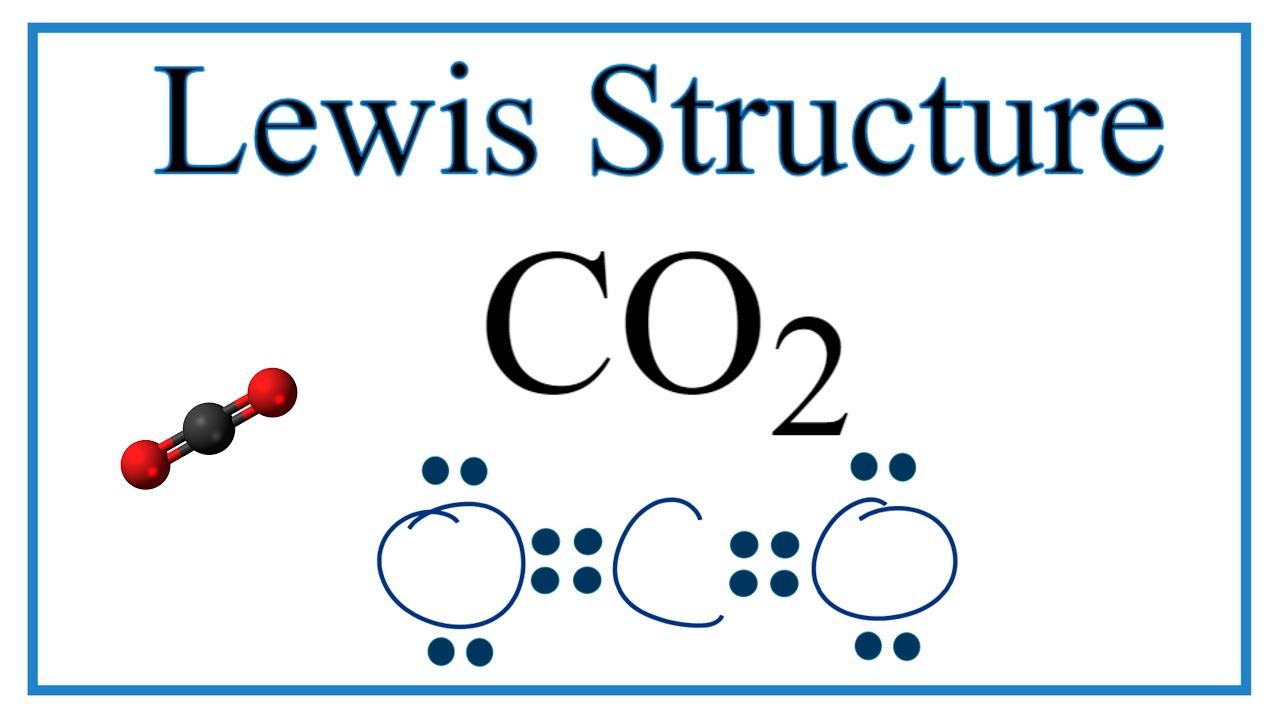
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. Lewis Dot Structure For Oxygen - Smart Lewis dot diagram can be drawn for element simple ions polyatomic ions ionic and molecular compounds. When drawing the structure you may replace the individual lines with two dots symbolizing the two electrons contained within the. Since Oxygen is in Period 2 it can fit a maximum of eight 8 electrons second energy level. CO2 Lewis Structure, Molecular Geometry and Hybridization CO2 Lewis Structure · Valence electrons in Carbon: 4 · Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we ...Feb 3, 2021 · Uploaded by Geometry of Molecules how to write co2 - Lisbdnet.com 37 CO2 Lewis Structure - How to Draw the Dot Structure for Carbon Dioxide; 38 Bài toán CO2 tác dụng với dung dịch kiềm|Thầy Phạm Thắng|TYHH; How To Write Co2? For example, write the formula for carbon dioxide as CO2, not CO2 or CO2. When writing formulae for ions, write the charge as a superscript.
› co2-lewis-structureCO2 Lewis Structure | Lewis Dot Structure For Molecules | UO ... Oct 17, 2021 · CO2 Lewis Structure. Now we learn how to draw a CO2 lewis structure, first, we find the total number of electrons by using the formula of Q. Q = Valance electron of all-atom + no of -ve charge – no of +ve charge . Q = 16 + 0 – 0. Q = 16. B.P e – = 2 × no of bonds. B.P e – = 2 × 2. B.P e – = 4 e – L.P e – = Q – B.P e – L.P e – = 16 – 4. L.P = 12
Draw the Lewis electron dot diagram for carbon dioxide on ... Which of the following is true regarding the Lewis diagram for carbon dioxide e? a. Carbon dioxide contains one central carbon atom covalently bonded to two oxygen atoms. Both of the bonds between carbon and oxygen are double bonds. b.Carbon dioxide contains one central carbon atom covalently bonded to two oxygen atoms.
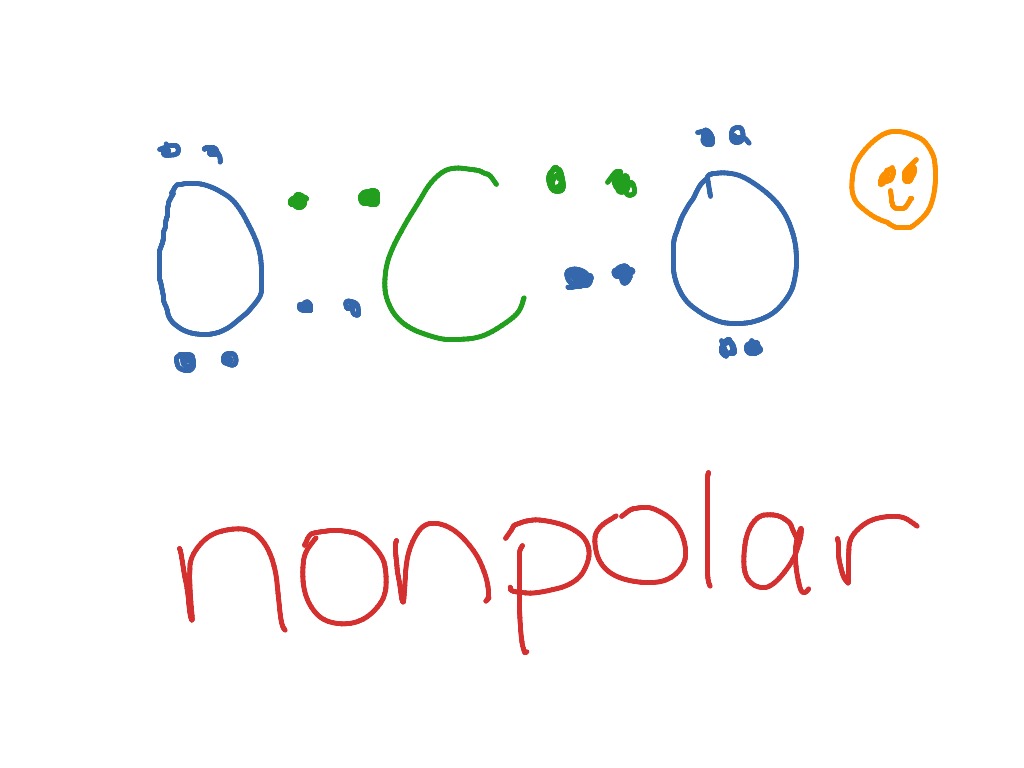
sciencetrends.com › co2-carbon-dioxide-lewis-dotCO2 (Carbon Dioxide) Lewis Dot Structure - Science Trends The Lewis Dot Structure for carbon dioxide can be represented like this: o=C=o. But what exactly does this mean? What is a Lewis Dot Structure, and what do the symbols in carbon dioxide’s structure represent? Let’s go over the Lewis structure and find out how to interpret this representation of carbon dioxide. How To Read A Lewis Dot Structure
Lewis Structure Of Cof2 - aunitedkingdomfilm.com Draw a Lewis structure for the following. In CoF2 crystal lattice in which eachCu2 ion is surrounded by six F- iconstwo of the sixF- ions immediately surrounding the Cu2 ion are distance of 227 A and the remaining four ions are at distance of 193 A The distortion thus leads to trigonal geometry.
How Many Unpaired Electrons Are In Co2? | iLoveMyCarbonDioxide Is CO2 capable of having tpaired electrons? The electrons in carbon are four because it is in group 4A of the periodic table. A covalent bond can be formed with four unpaired electrons in carbon. As shown in the illustration, the CO2 molecule is represented by a Lewis dot structure.
Lewis Dot Structure Generator - Summarized by Plex.page ... Lewis Dot form of gold would be Au sign for gold with single dot. Lewis dot layout for neon has a set of electrons on each side of the neon icon, Ne, for a total of 8 electrons. Asked in Chemistry, Lewis dot layout for Li?A Lewis structure is a structural representation of particle where dots are utilized to reveal electron placement around atoms.
Lewis Structures: Single, Double & Triple Bonds - Video ... Lewis Dot Structure for Compounds. Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows ...
CO2 Lewis Dot Structure - RDE-34 Here is the answer for the question - CO2 Lewis dot structure. You'll find the correct answer below Content Table CO2 Lewis dot structure Reason Explained CO2 Lewis dot structure The Correct Answer is Linear 180 Reason Explained Linear 180 is correct for CO2 Lewis dot structure Alex Timmons
Carbonate Lewis Structures - carbonate Draw the Lewis structure for carbonate ion CO2 3 CO 3 2. One of these oxygen atom take a proton H ion and form a -OH group. For this molecule C will appear as the central atom since its less electronegative than O. CO32- Lewis Structure - How to Draw the Lewis Structure for CO3 2- Carbonate Ion - YouTube. Lewis Structure of CO2.
Lewis Dot Diagram for CO2 - How To Discuss Draw the Lewis dot diagram for CO2 by identifying the lone pairs. Check the octal rule and atom charges It is important to note any charges on the atoms shown in the above CO2 structure. Each oxygen and carbon atom does have charges, as seen in the following image. It is critical to know the charges to arrive at the finest Lewis structure possible.
› watch › dD0Xl4acUFgCO2 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number ...
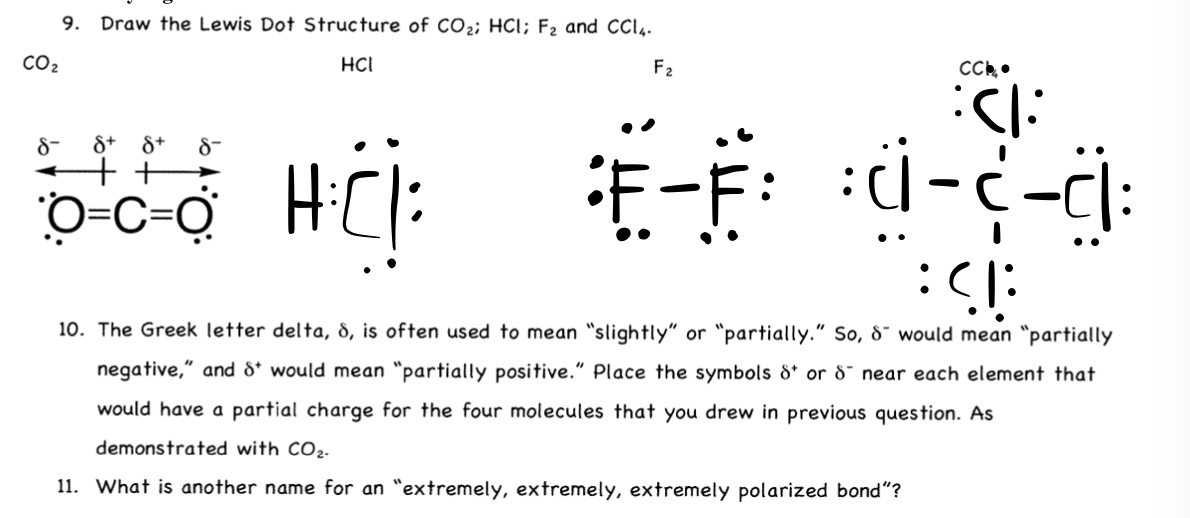
Dipoles & Dipole Moments: Molecule Polarity - Video ... You will need the Lewis dot structure for CH 3 Cl. For each of the carbon-hydrogen bonds, I draw an arrow pointing towards carbon. For the carbon-chlorine bond I draw an arrow pointing towards ...
What Is The Correct Lewis Structure For Co2 20) The correct Lewis structure for CO2 shows that the molecule contains two double bonds. Finally, Why does the correct Lewis structure of CO2?, Carbon dioxide has a total of 16 valence electrons, 4 from carbon and 6 from each of the two oxygen atoms. In order to give the central carbon atom a complete octet, you need to form two double bonds ...
Carbon Dioxide Dot And Cross Diagram - Summarized by Plex ... The first step to drawing the Lewis Structure is to determine how many valence electrons a molecule has in total. This involves counting up all valence electrons in molecule. Valence electrons are electrons an atom possesses in its license shell or outermost shell of the atom.
Lewis Dot Structure For Co2 - How To Discuss Lewis Dot Structure For Co2 How do you represent the Lewis point structure for CO2? Carbon (C) is the least electronegative atom in the Lewis CO2 structure and therefore must be placed in the center of the structure. The Lewis structure for CO2 has a total of 16 valence electrons.
› how-to-draw-lewisHow To Draw Lewis Structure For Co2? | iLoveMyCarbonDioxide Nov 25, 2021 · What Is The Lewis Diagram For Co2? The Lewis structure of carbon dioxide (CO) consists of two oxygen atoms and one carbon atom. The carbon atom in the CO has two double bonds. The valence shells of carbon atoms and oxygen atoms each contain two lone pairs. Linearity is the characteristic of CO.
wellcometreeoflife.org › co2-lewis-structureCO2 Lewis Structure (2021 UPDATED) All You Need To Know Dec 22, 2021 · The number of valence electrons of a carbon atom is four which forms four bonds. The CO2 Lewis Structure is where the central carbon atom is the central atom, the least electronegative atom with O surrounded by four dots. The total valence electrons, including valence shells, are eight electrons pairs with no lone pair.
what is the shape of carbon dioxide - Lisbdnet.com What is the dot structure of carbon dioxide? CO2 Lewis Structure Setup. Carbon has four valence electrons that form a total of four bonds. So carbon is shown with four dots around it. Oxygen needs just two bonds, represented as the lone dots to the left and right of the O atoms. The pairs of dots above and below the O's won't bond.








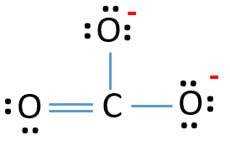













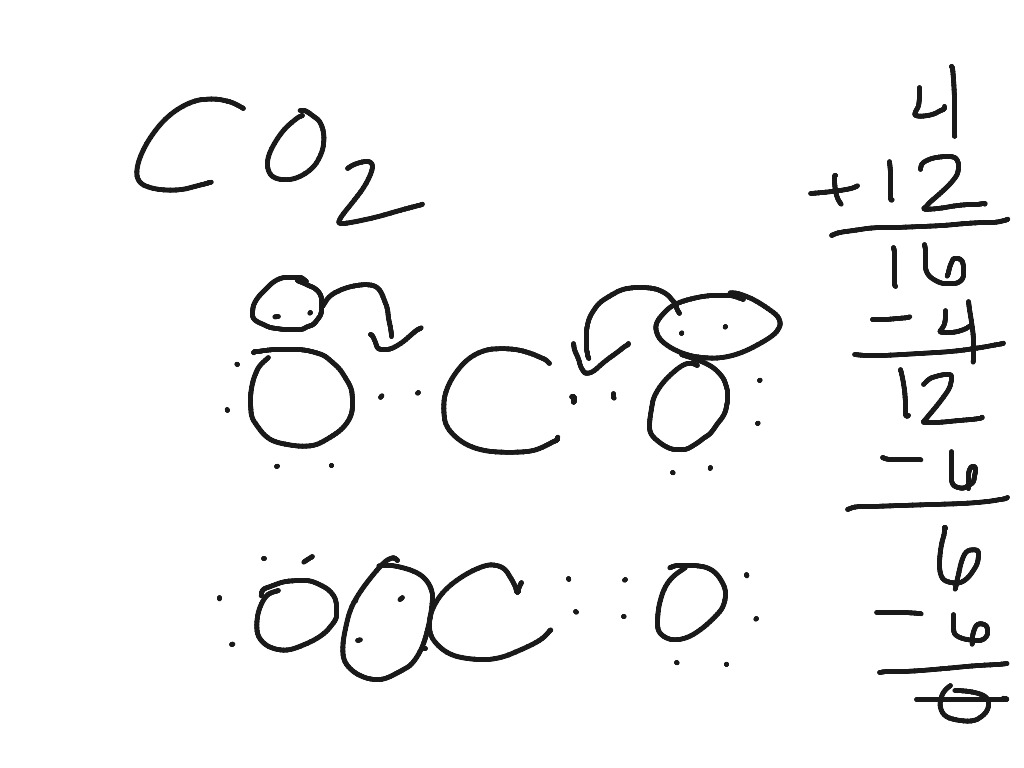
Comments
Post a Comment