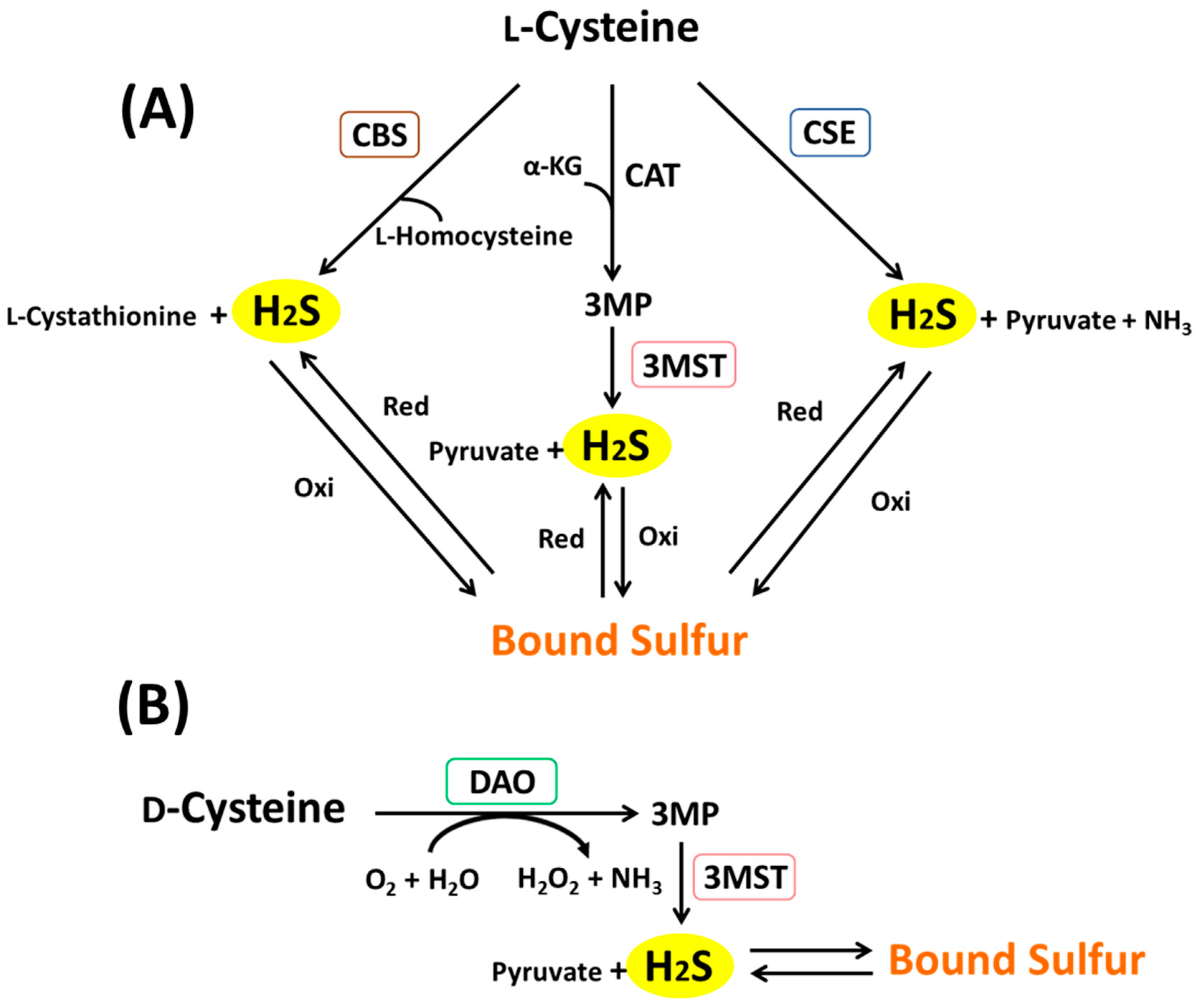
43 sulfur atom diagram
Atomic Structure Worksheet - Washoe County School District Give the symbol and number of electrons in a neutral atom of: Uranium Chlorine Boron Iodine Antimony Argon Give the isotope symbol and number of neutrons in one atom of the following elements. Show your calculations. Barium – 138 Sulfur – 32 Carbon – 12 Hydrogen – 1 Building a 3D Atom Structure of Sulfur - Chemistry ... Building a 3D Atom Structure of Sulfur. August 26, 2021. Erwin van den Burg. 6 min read. Table of Contents: 8.6: Exceptions to the Octet Rule; Lewis Structures; The Expanded Octet; Space-filling model. This article is about a topic in chemistry. For space-filling curves in geometry, see Space-filling curve. ... Video advice: Elementary 4 ...
SF2 Lewis Structure, Molecular Geometry, Hybridization ... The electronic configuration of Sulfur is 1s2 2s2 2p6 3s2 3p4. First, the electrons are filled in 1s, then in 2s, and so on. Similarly, the electronic configuration of Fluorine is 1s2 2s2 2p5. These configurations are decided on the basis of the number of electrons these elements have.

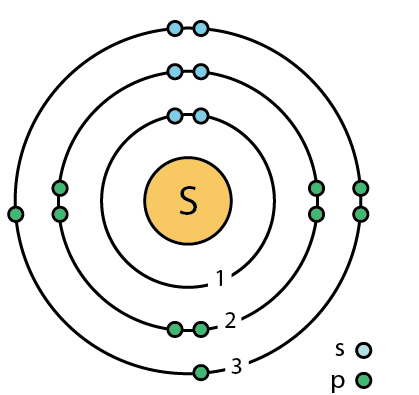
Sulfur atom diagram
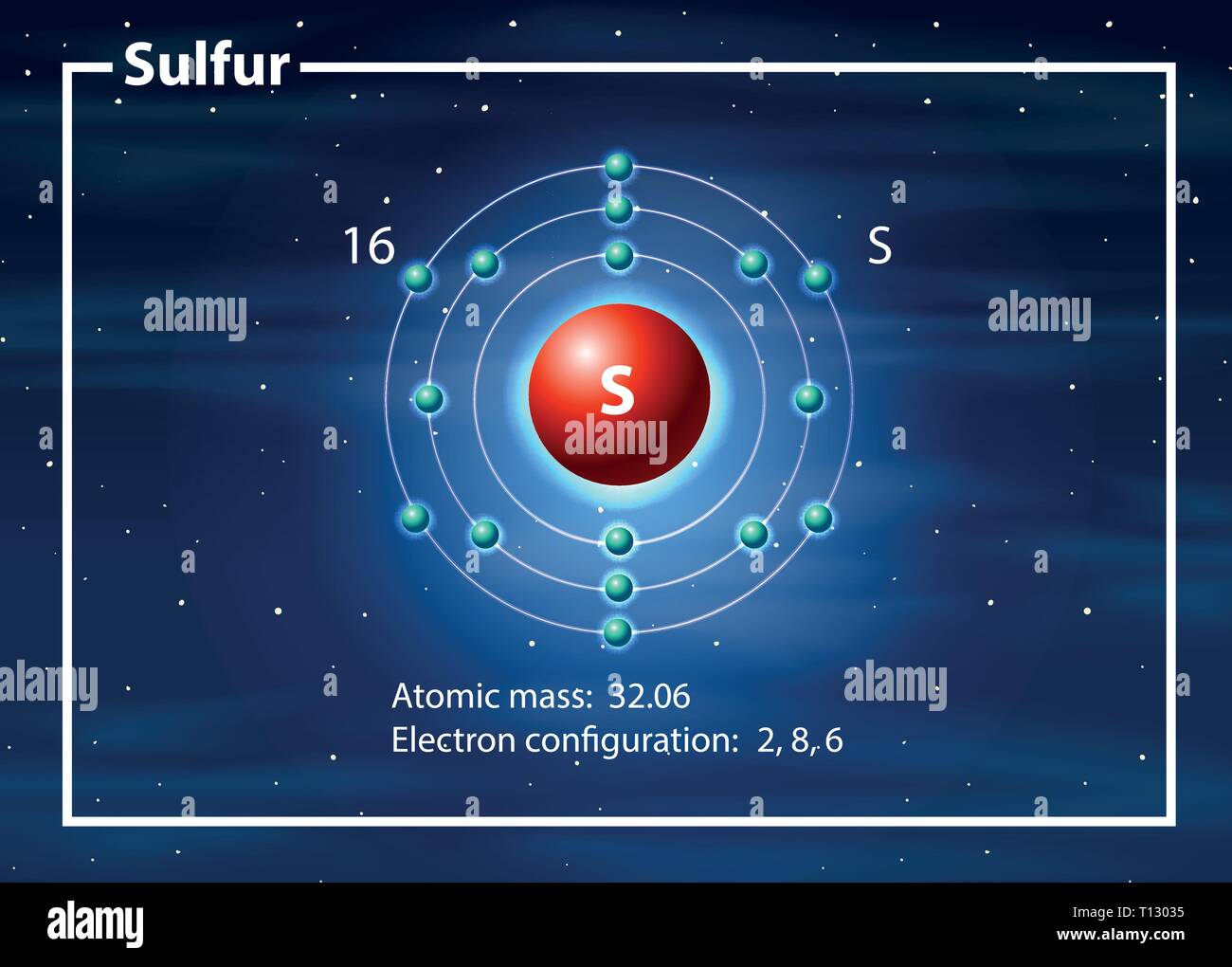
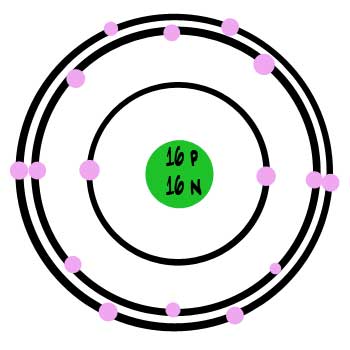
Sulfur - Element information, properties and uses ... Element Sulfur (S), Group 16, Atomic Number 16, p-block, Mass 32.06. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. ... The number of protons in an atom. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. ... ChemSpider is a free chemical structure database ... Lewis Dot Structure for Sulfur Atom (S) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ... S Sulfur Element Information: Facts, Properties, Trends ... Sulfur or sulphur (see spelling differences) is a chemical element with symbol S and atomic number 16. It is an abundant, multivalent non-metal. Under normal ...Electronic Shell Structure: 2, 8, 6Atomic Weight: 32.065Atomic Number: 16Electronic Configuration: 3s2 3p4
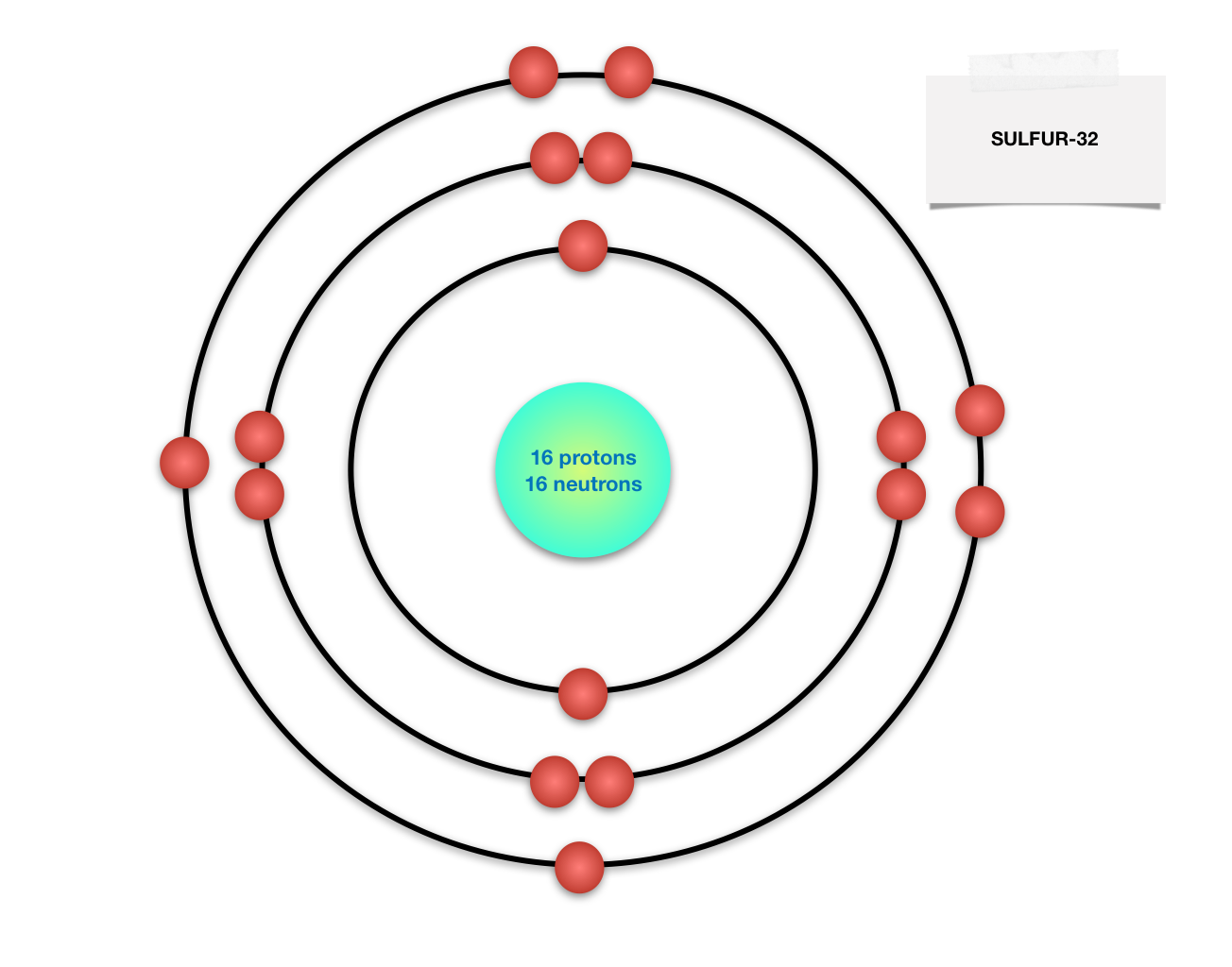
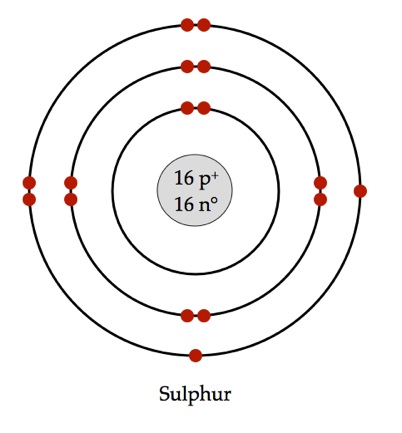
Sulfur atom diagram. Sulfur stock illustration. Illustration of structure ... Sulfur. Illustration about structure, composition, neutrons, atoms, chemical, isolated, white, compound, sulfur, background, protons, science, educational, atomic, symbol - 83614882 ... 3d render of atom structure of sulfur isolated over white background Protons are represented as red spheres, neutron as yellow spheres, electrons as blue ... Emission spectrum - Wikipedia The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for … How to draw SO3 Lewis Structure? - Science Education and ... In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Sulfur, atomic structure - Stock Image - C018/3697 ... Sulfur (S). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of sulfur-32 (atomic number: 16), the most common isotope of this element. The nucleus consists of 16 protons (red) and 16 neutrons (orange). 16 electrons (white) occupy available electron shells (rings).
Sulfur | S - PubChem Topical preparations containing sulfur are intended for external use only. Topical sulfur-containing preparations should not be used near the eyes; ...If topical sulfur-containing preparations are used for self-medication and the condition worsens or persists after regular use as directed, a physician should be consulted.If excessive skin irritation develops or increases during self-medication ... Sulfur and Phosphorus Compounds.docx - Sulfur and ... The nomenclature of sulfur compounds is generally straightforward. The prefix thio denotes replacement of a functional oxygen by sulfur. Thus, -SH is a thiol and C=S a thione. The prefix thia denotes replacement of a carbon atom in a chain or ring by sulfur, although a single ether-like sulfur is usually named as a sulfide. For example, C 2 H 5 SC 3 H 7 is ethyl propyl sulfide and C 2 H 5 SCH ... Bohr Diagram For Sulfur The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. How to Make a Model of a Sulfur Atom. High-Pressure Mg–Sc–H Phase Diagram and Its ... To determine the ternary phase diagram of the Mg–Sc–H system under high pressure, ... with energy differences of 0.003, 0.032, 0.008, and 0.015 eV/atom, respectively. ... which are reminiscent of the trigonal phase of sulfur. The R‾3m-SrH6 phase, which is …
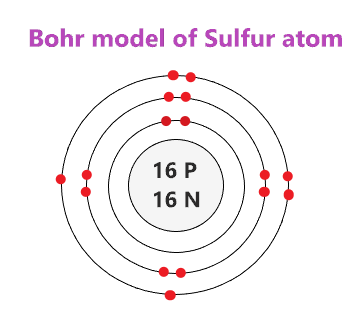
Sulfur Bohr Model - How to draw Bohr diagram for Sulfur (S ... Here, we will draw the Bohr diagram of the Sulfur atom with some simple steps. Steps to draw the Bohr Model of Sulfur atom. 1. Find the number of protons, electrons, and neutrons in the Sulfur atom. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Assign an oxidation number to each element ... - Brainly.com 02-03-2018 · Although Sulfur in the compound is also a single element, but as Calcium comes first, therefore, we would consider Ca as an independent element. Hence, Ca has the oxidation number +2. Blank 2: As I mentioned before, the net charge on the CaSO4 is zero; therefore, the sum of the oxidation number of Ca, S and that of O4 has to be zero. Question Video: Deducing the Energy Level Diagram ... The atomic number tells us the number of protons a sulfur atom contains in its nucleus. As a neutral atom, sulfur will also contain the same number of electrons. Therefore, a sulfur atom will have a total of 16 electrons. Energy level K will hold the first two electrons. The next energy level L will hold the next eight electrons in the sulfur atom. Blank Atom Diagram - Fill and Sign Printable Template ... What is the electron configuration of a sulfur atom in the ground state 1 2 4 2 2 6 3 2 8 4 4 2 8 6 2. A neutral atom always has an equal number of 1 neutrons and electrons 2 neutrons and protons 3 protons and electrons 4 protons electrons and neutrons. 3. Below is a Bohr-Rutherford diagram of an element.
Bohr Diagram Of Sulfur - schematron.org Sulfur (S). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of sulfur (atomic number: 16), the most.T. Trimpe schematron.org Atomic Basics Answer Key Part A: Atomic Structure 1. Draw five protons in the nucleus of the atom. Label them with their charge. How to Make a Model of a Sulfur Atom.
Hydrodesulfurization - Wikipedia Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide (SO
Atom Diagrams: Electron Configurations of the Elements An ion of an atom is one in which the number of protons and electrons is not the same. If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. If there are more electrons than protons, the ion has a negative charge and is called an anion. Elements are shown from atomic number 1 (hydrogen) up to 94 ...
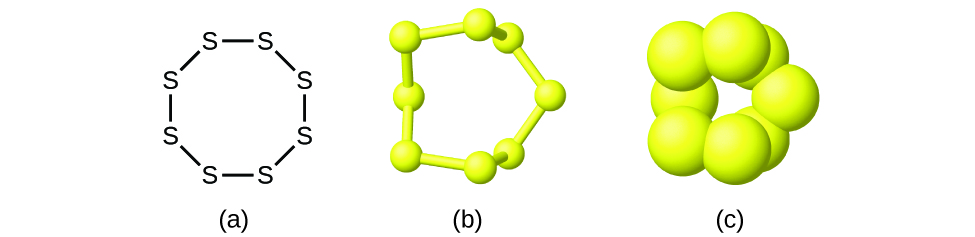
Sulfur - Wikipedia Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8.Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the ...
Solved Question 1 (10 points) When you diagram an atom of ... Chemical Engineering questions and answers. Question 1 (10 points) When you diagram an atom of sulfur you have A/ electrons in the first row, A/ electrons in the second row and A/ electrons in the third row. The answers are all numbers. Question: Question 1 (10 points) When you diagram an atom of sulfur you have A/ electrons in the first row, A ...
Bohr Diagram Of Sulfur - wiringall.com Sulfur (S). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of sulfur (atomic number: 16), the most. In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,.
Sulfurous Acid (H2SO3) Lewis Structure H 2 SO 3 lewis structure. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure. Steps of drawing lewis structure of H 2 SO 3. There are several steps to draw the lewis structure of H 2 SO 3. Those ...
How would you explain the phase diagram of sulfur class 11 ... The two solid phases of the sulfur atom are monoclinic and rhombic form. The phase diagram of sulfur is given below: - There are three triple points of sulfur and it is indicated in the diagram with numbers 1, 2, and 3. The most stable form of a sulfur element is the rhombic form and when it is heated slowly then the rhombic form will convert ...
Sulfur Orbital diagram, Electron configuration, and ... The Sulfur orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining four electrons in 3p orbital. Orbital diagram for a ground-state electron configuration of a Sulfur atom is shown below- What is the electron configuration of the S2- ion?
Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
Bohr Diagram For Sulfur - schematron.org Its atomic symbol is Cu. Each atom has 29 protons and electrons. The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not. Here we go: 1st energy level - 2 electrons max 2nd energy level - 8 electrons max 3rd+ levels - 18 electrons max.
Sulfur(S) electron configuration and orbital diagram Sulfur (S) excited state electron configuration and orbital diagram This electron configuration shows that the last shell of the sulfur atom has six unpaired electrons (3s 1 3p x1 3p y1 3p z1 3d xy1 3d yz1 ). So the valency of sulfur is 6. From the above information, we can say that sulfur exhibits variable valency.
How to draw SBr2 Lewis Structure? - Science Education and ... In the SBr2 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SBr2 Lewis structure, with all two bromine atoms arranged in a tetrahedral geometry. Add valence electrons around the bromine atom, as given in the figure.
What Is the Orbital Diagram for Sulfur? - Reference.com The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
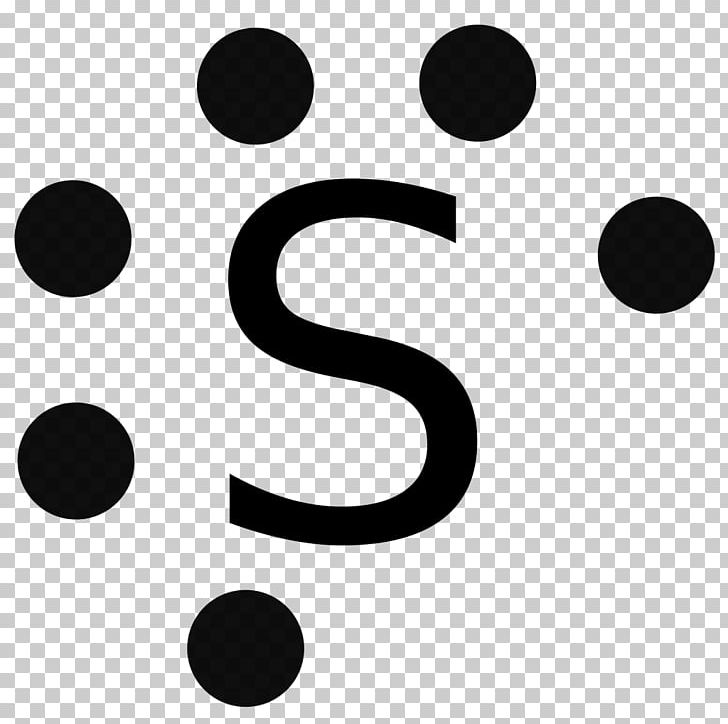
Electron dot diagram for sulfur? - Answers The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1). Wiki User ∙ 2011-01-28 05:59:12
Lewis Structure of SO2 (2021 UPDATED) Complete Guide A sulfur atom on the outer level has six electrons, and four are used by Oxygen for each bond. That leaves a total number of ten electrons in five pairs. One lone pair is left while two double bonds form as a unit, making the Bent or V shape. Molar Mass You can calculate the molar mass by using the usual formula n=N/NA (then N=n*NA).
Electron Configuration for Sulfur (S) - UMD In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
S Sulfur Element Information: Facts, Properties, Trends ... Sulfur or sulphur (see spelling differences) is a chemical element with symbol S and atomic number 16. It is an abundant, multivalent non-metal. Under normal ...Electronic Shell Structure: 2, 8, 6Atomic Weight: 32.065Atomic Number: 16Electronic Configuration: 3s2 3p4
Lewis Dot Structure for Sulfur Atom (S) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
Sulfur - Element information, properties and uses ... Element Sulfur (S), Group 16, Atomic Number 16, p-block, Mass 32.06. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. ... The number of protons in an atom. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. ... ChemSpider is a free chemical structure database ...


:max_bytes(150000):strip_icc()/lithiumatom-58b602933df78cdcd83d927b.jpg)





















Comments
Post a Comment