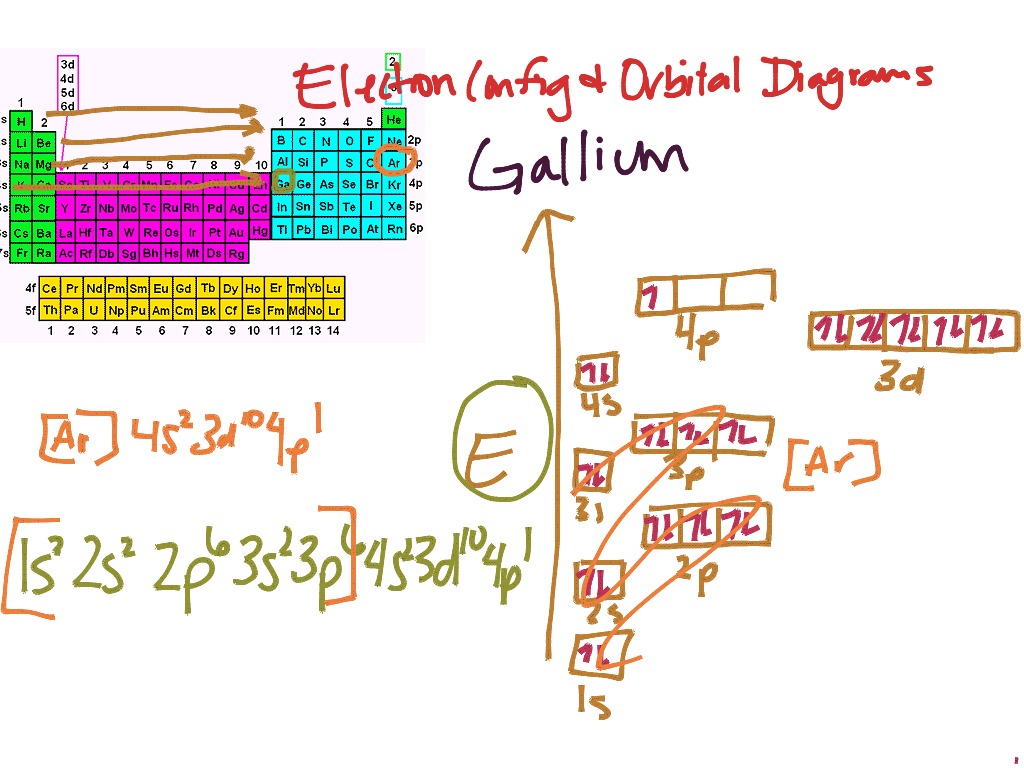
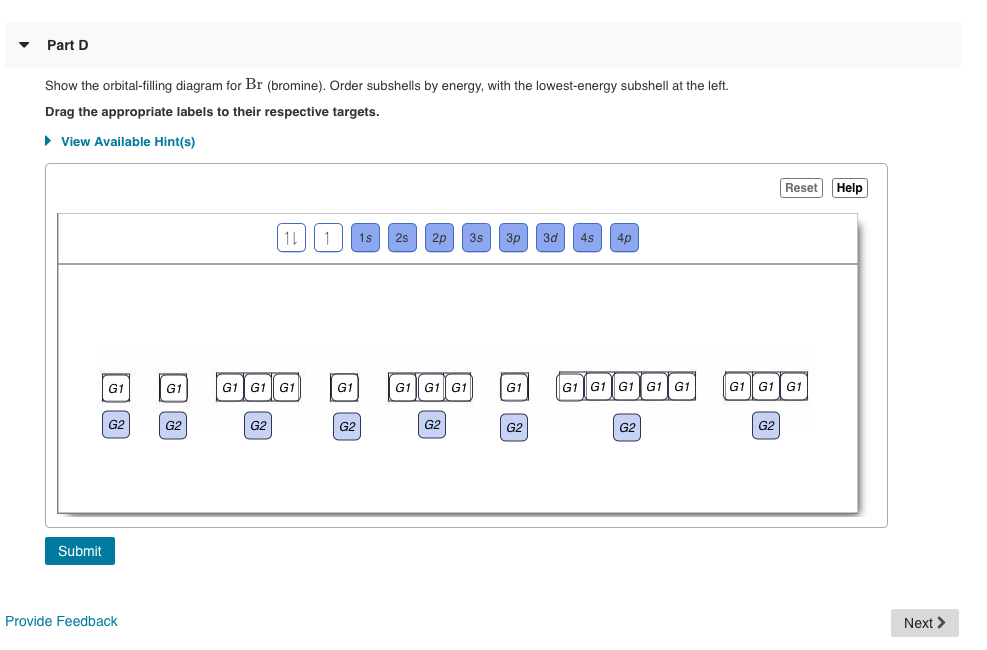
43 Orbital Diagram For Br
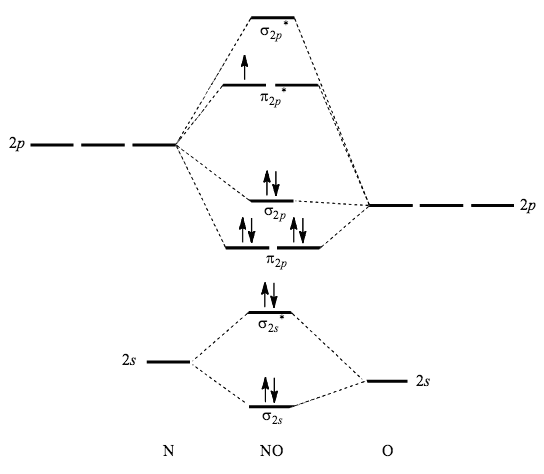
Orbital Neoplasms in Adults: Clinical, Radiologic, and ... Oct 01, 2013 · Orbital neoplasms in adults may be categorized on the basis of location and histologic type. Imaging features of these lesions often reflect their tissue composition. Cavernous malformations (also known as cavernous hemangiomas), although not true neoplasms, are the most common benign adult orbital tumor. They typically appear as a well-circumscribed, ovoid … Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
Electron configuration for Molybdenum (element 42 ... Orbital diagram. Molybdenum electron configuration ← Electronic configurations of elements . Mo (Molybdenum) is an element with position number 42 in the periodic table. Located in the V period. Melting point: 2617 ℃. Density: 10.28 g/cm 3. The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. ...
Orbital diagram for br
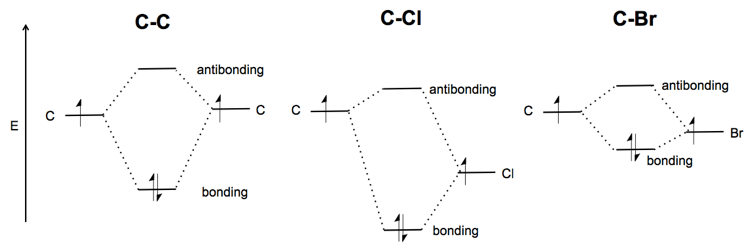
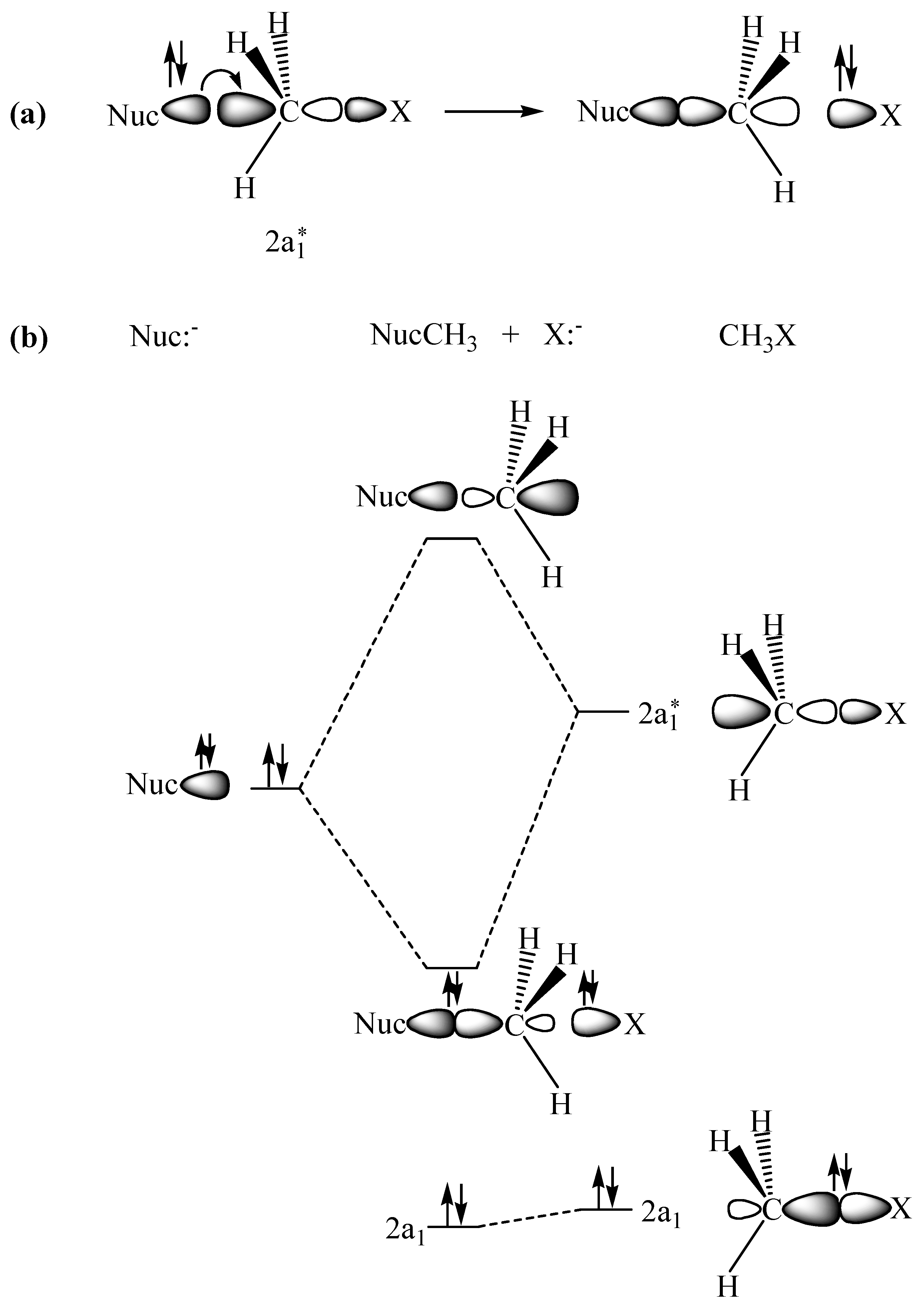
Quantum Numbers and Electron Configurations The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9. Molecular Structure & Bonding - Chemistry The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond. Orbital Apex Syndrome: A Review Dec 12, 2019 · Orbital apex syndrome can also develop due to compression by neural tumors in the orbital apex. Meningiomas, schwannomas, and neurofibromas can cause compression of the contents of the orbital apex. A rare case of orbital apex syndrome after initiating treatment of skin melanoma with Ipilimumab.
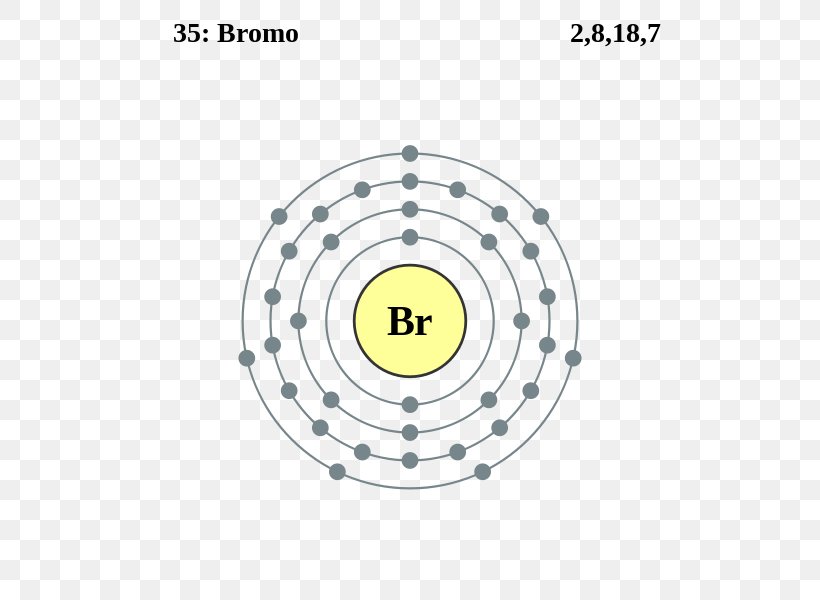
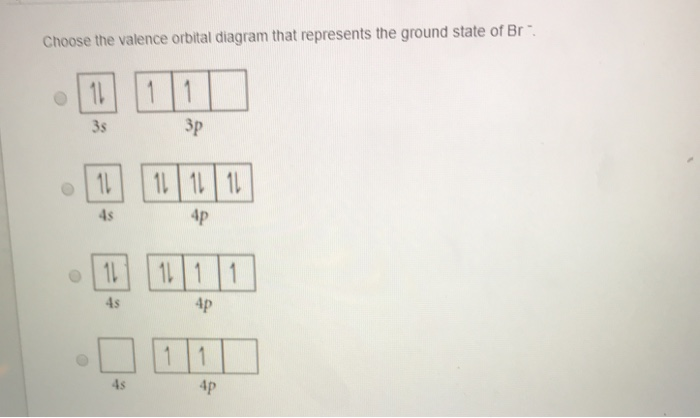
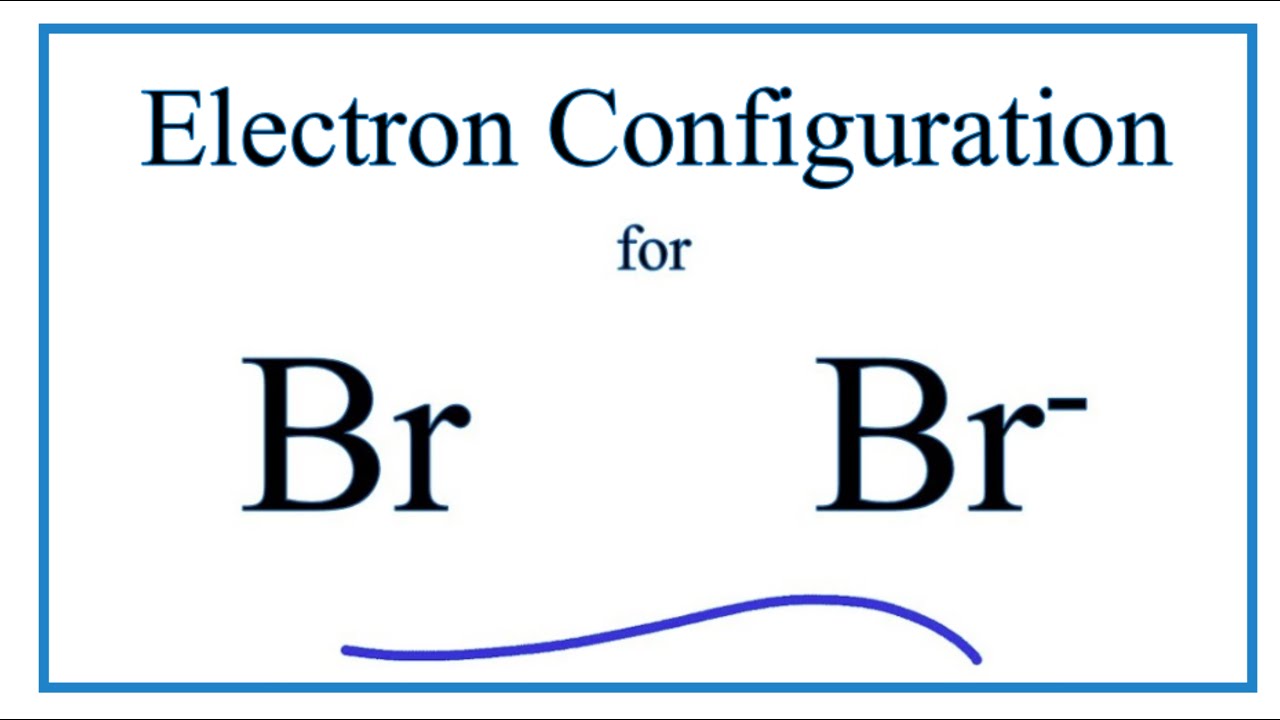
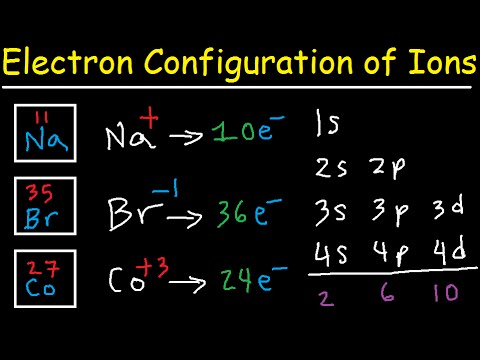
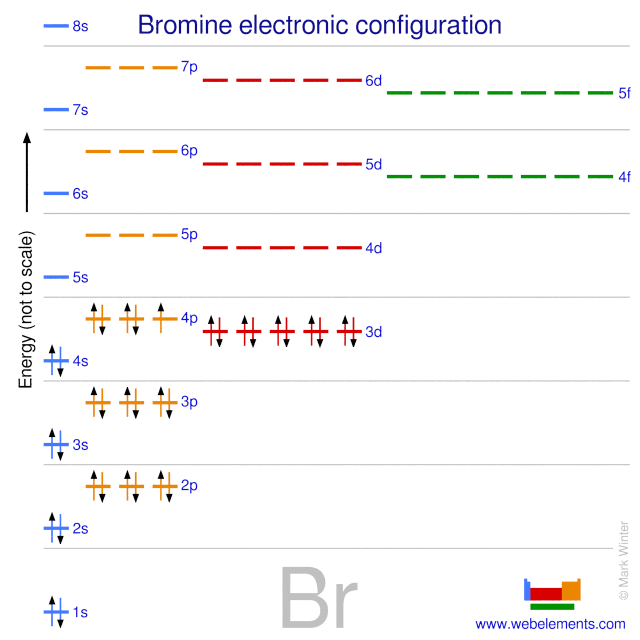
Orbital diagram for br. Miessler-Fischer-Tarr5e SM Ch 05 CM 5.12 a. The KrBr+ energy level diagram is at the right. b. The HOMO is polarized toward Br, since its energy is closer to that of the Br 4p orbital. c. Bond order = 1 d. Kr is more electronegative. Its greater nuclear charge exerts a stronger pull on the shared electrons. 4s 4s Kr KrBr+ Br 4p 4p HOMO 2s 2p 2s 2p N NF F 2s 2s * Bromine(Br) electron configuration and orbital diagram The 3d orbital is now full. So, the remaining five electrons enter the 4p orbital. Therefore, the bromine(Br) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. How to write the orbital diagram for bromine(Br)? To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Neon(Ne) electron configuration and orbital diagram To write the orbital diagram of neon(Ne), you have to do the electron configuration of neon. Which has been discussed in detail above. Neon (Ne) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund’s principle, the first electron will enter in ... Orbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12 ...
Orbital Apex Syndrome: A Review Dec 12, 2019 · Orbital apex syndrome can also develop due to compression by neural tumors in the orbital apex. Meningiomas, schwannomas, and neurofibromas can cause compression of the contents of the orbital apex. A rare case of orbital apex syndrome after initiating treatment of skin melanoma with Ipilimumab. Molecular Structure & Bonding - Chemistry The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond. Quantum Numbers and Electron Configurations The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.

/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)





















Comments
Post a Comment