40 molecular orbital diagram practice
Practice Test Questions 4 . Molecular Orbital Theory: Polyatomic Molecules . 1. The images below show the valence molecular orbitals obtained for the carbonate ion via a semi - empirical calculation. Both side views and top views are provided, and each MO has been assigned an identifying letter. Using Symmetry: Molecular Orbitals One approach to understanding the electronic structure of molecules is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule.
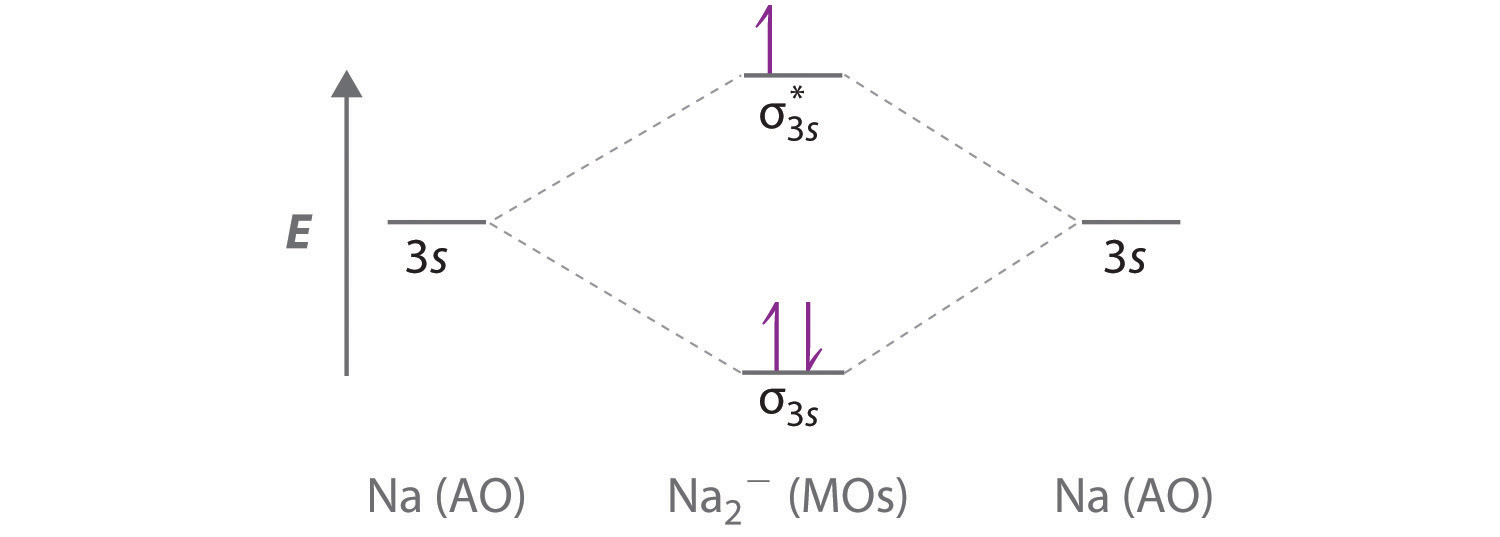
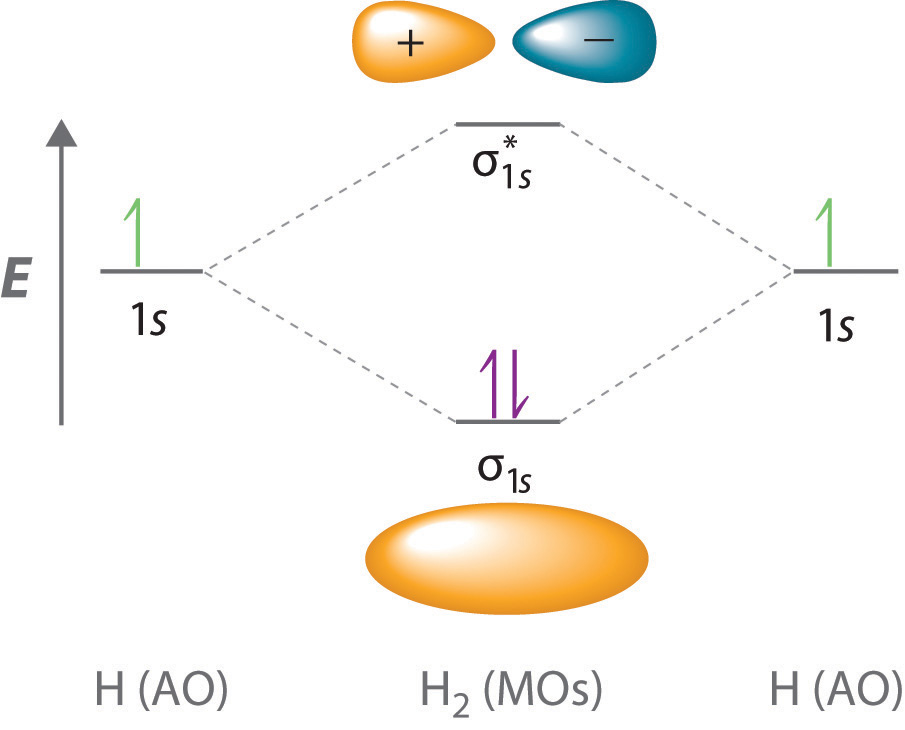
Answers to Practice Test Questions 3 . Molecular Orbital Theory: Heteronuclear Diatomic Molecules . 1. (a) 1The electron configuration for 𝐻𝐻 is 1𝑠𝑠, so 𝐻𝐻 has 1 valence electron. The electron configuration for 𝐻𝐻𝐻𝐻 is 1𝑠𝑠2, so 𝐻𝐻𝐻𝐻 has 2 valence electrons.
Molecular orbital diagram practice
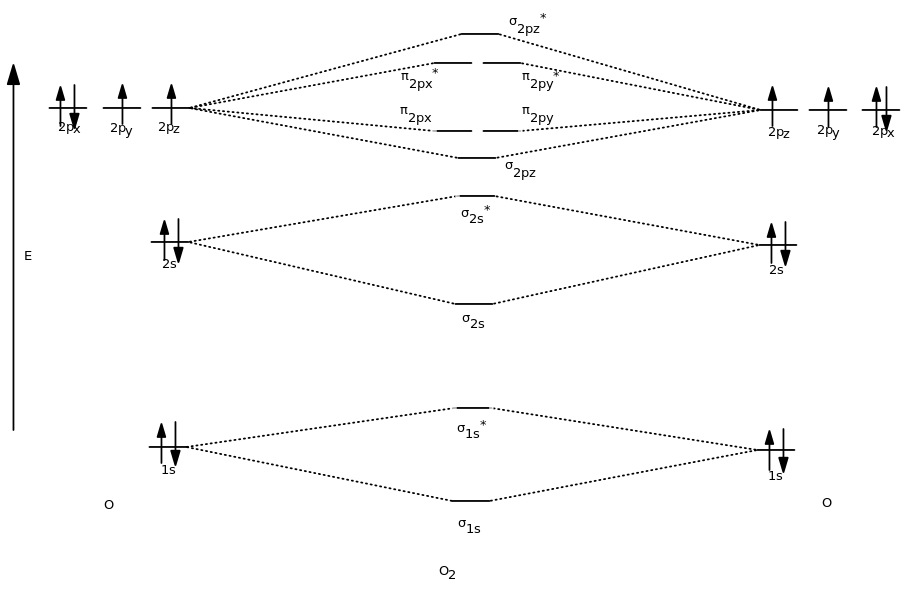
Practice Problems Molecular Structure and Covalent Bonding. 1. Write Lewis structures for (a) XeF 4, (b) ... Draw a molecular orbital diagram for the hypochlorite ion. What is the bond order? Is this consistent with the bonding predicted by a Lewis dot structure? 17. Draw a molecular orbital diagram for the hydroxide ion. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Feb 15, 2015 · By Marsha Massey. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules. The site includes opportunities to practice filling in electrons, attaching the names/symbols of MOs, and matching orbital overlap drawings to MOs.
Molecular orbital diagram practice. Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Multiple choice questions by Catherine E. Housecroft. This activity ... In an MO diagram for the formation of H2O in which the z axis bisects the H-O-H angle: .... MCQ quiz on Chemistry multiple choice questions and answers on. Chemistry MCQ questions quiz on ... Theories | Molecular Orbital
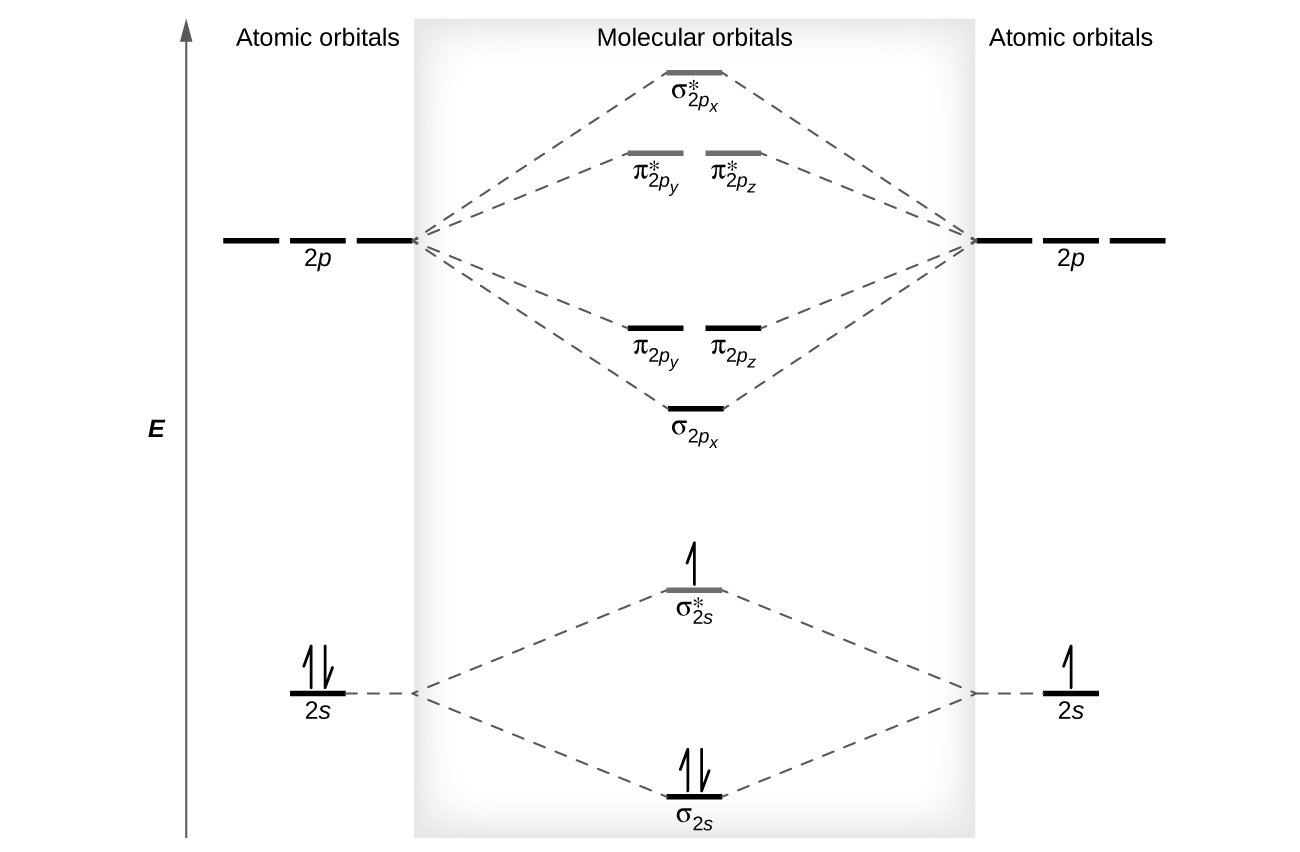
Figure 7.7.9. This is the molecular orbital diagram for the homonuclear diatomic Be 2+, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals, using the Aufbau principle and Hund's rule. Bond Order Problem: Draw a molecular orbital diagram for Ar2+. This ion has been observed in the gas phase. Calculate bond order and describe how the bond distance in this ion would differ from that in Cl2. Draw a molecular orbital diagram for Ar 2+. This ion has been observed in the gas phase. These problems are for practice in drawing your molecular orbital diagrams, molecular electron configurations and determining bond order. The following questions pertain to the F2 molecule: A) Draw the molecular orbital energy diagram for this molecule. Label all of the orbitals specifically. *2px In this video, we do some practice with the molecular orbitals of molecules by linear combinations of 2s and 2p orbitals. Remember, there is one 2s orbital ...
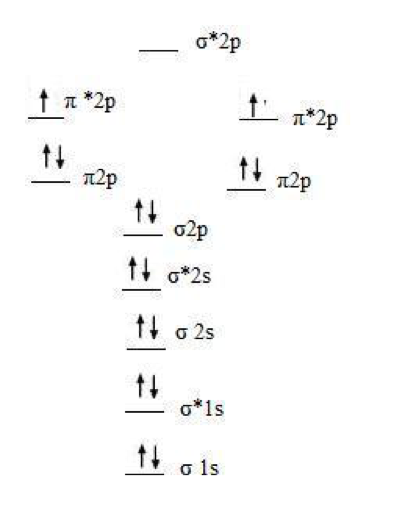
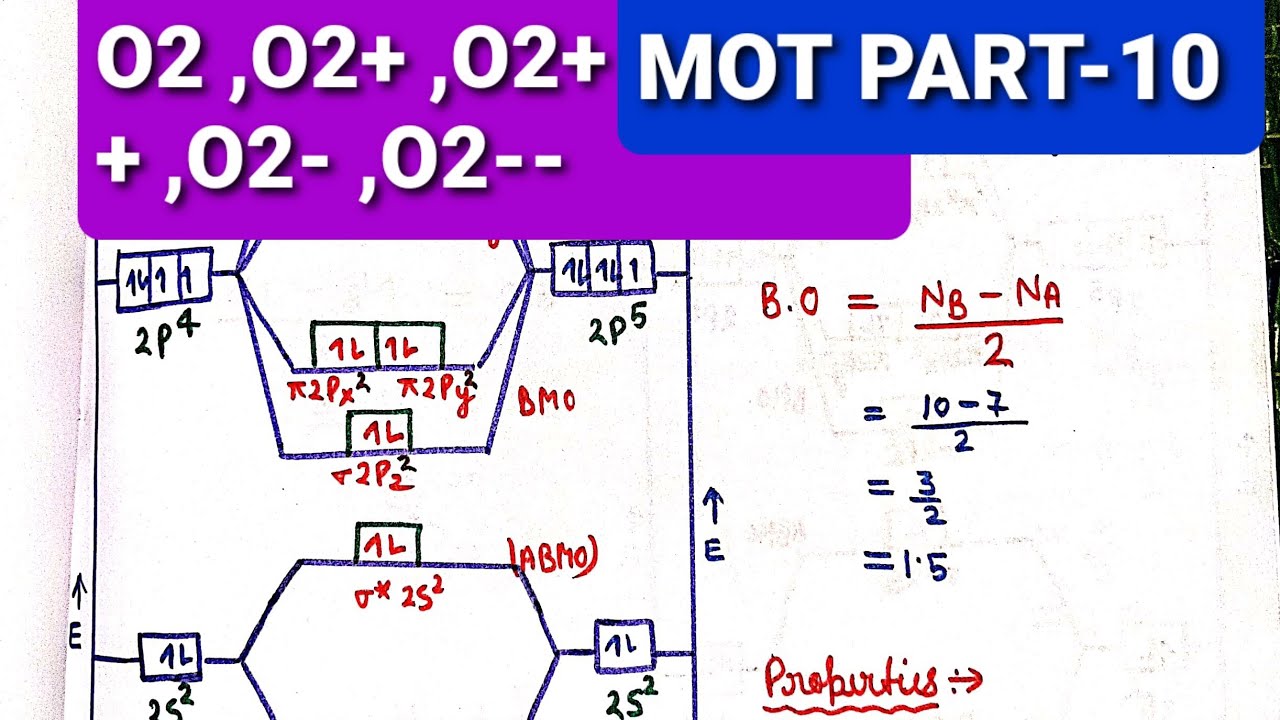
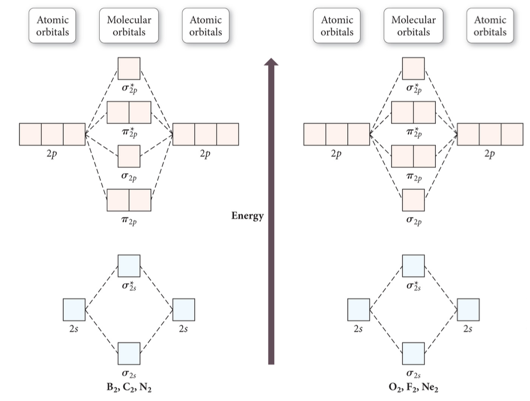
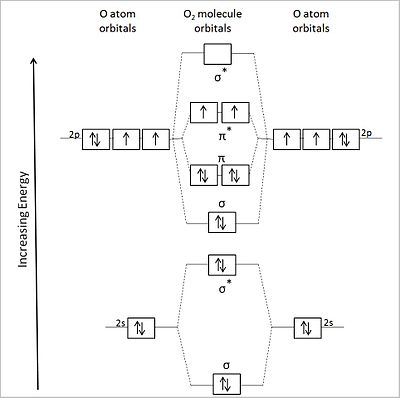
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. 1. Comment actions. Permalink. I would also greatly appreciate a series on this topic. (Lewis,) VSEPR, Valence Orbitals and MO. In MO theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. Between molecules like N2, O2 and others like HF. Thanks so much!!! The number of molecular orbitals created by hybridization depends on the number of atomic orbitals that are mixed to form them. In forming sp3 hybridized orbitals, four atomic orbitals are mixed, one s and three p. The energy diagram for this process is shown below. The hybridized orbitals are higher in energy than the s By constructing a molecular orbital picture for each of the following molecules, determine whether it is paramagnetic or diamagnetic. a. B 2 b. C 2 c. O 2 d. NO e. CO a. B 2 is paramagnetic because it has two unpaired electrons, one in each of its p orbitals. b. C 2 is diamagnetic because all of its electrons are paired.
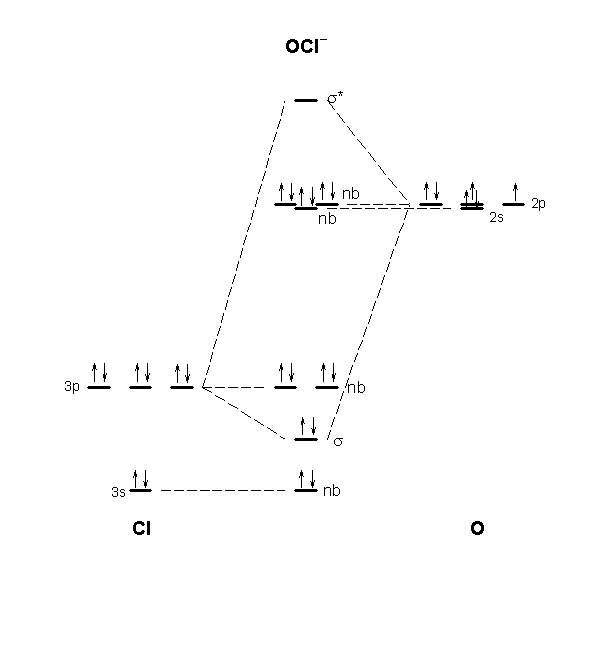
The molecular orbital diagram for ClO – is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
Molecular orbital Diagram Practice. molecular orbital diagrams of diatomics worksheet in chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule in this theory each ...
This lesson will help you: Understand the molecular orbital theory. Explain the assumption behind the theory. Identify how to determine the shape of electron orbitals. Describe how carbon can bond ...
Molecular Orbital Theory Practice Questions. Chemistry End of Chapter Exercises. Sketch the distribution of electron density in the bonding and antibonding molecular orbitals formed from two s orbitals and from two p orbitals. How are the following similar, and how do they differ? (a) σ molecular orbitals and π molecular orbitals ...
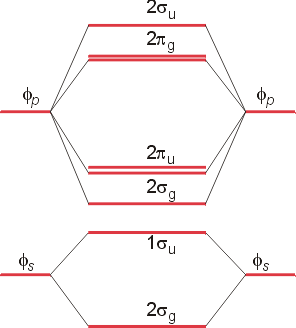
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Here are some boxes for you to practice drawing s orbitals in, although you do not really need boxes. As we proceed developing atomic and molecular orbit- ... For this we need to picture atomic and molecular orbitals. l = 0 2. ATOMIC ORBITALS 2p x 2p y 2p z l = 1 x y z n = 2 This is an accurate representation of a 2p x orbital. This is a common ...
Molecular orbital Diagram Practice. molecular orbital diagrams of diatomics worksheet in chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule in this theory each ...
Chapter 11 Answers Practice Examples 1a. There are three half-filled 2p orbitals on N, and one half-filled 5p orbital on I. Each half-filled 2p orbital from N will overlap with one half-filled 5p orbital of an I. Thus, there will be three N—I bonds. The I atoms will be oriented in the same direction as the three 2p orbitals of N: toward the x ,y, and z-directions of a Cartesian coordinate ...
(a) Complete the valence molecular orbital energy level diagram below by: i. drawing and naming the atomic orbitals, ii. drawing and naming the molecular orbitals, and iii. populating the atomic and molecular orbitals with electrons (b) Write the valence orbital occupancy (i.e. electron configuration) for − 2 2 C.
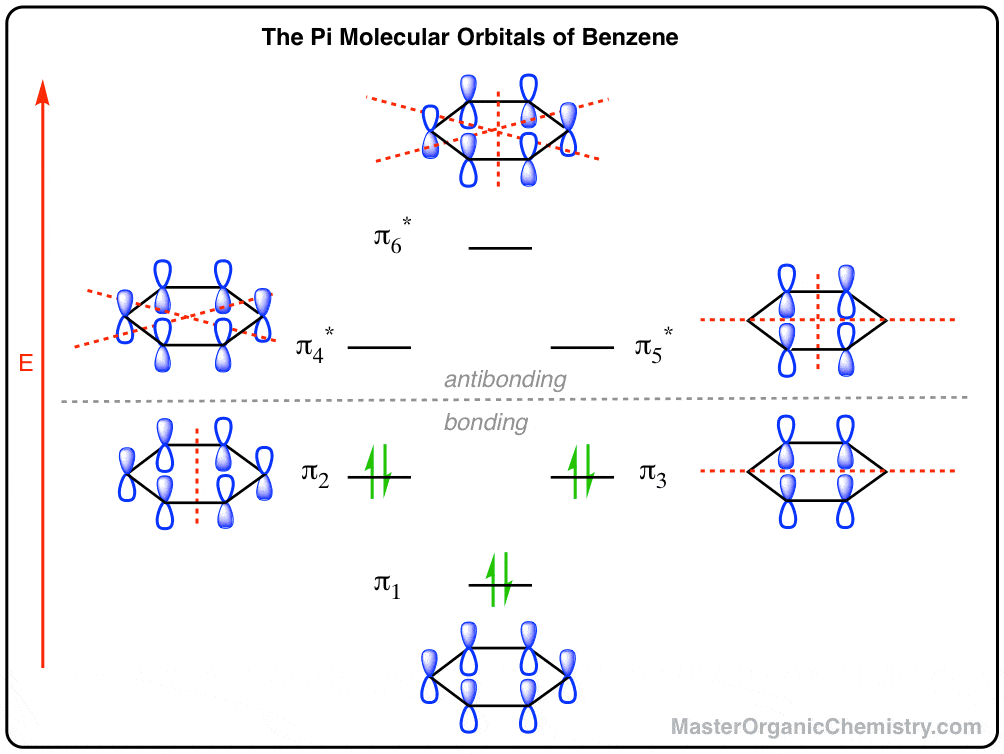
Nov 12, 2021 · Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are different, these molecular orbital diagrams will look incredibly different.
Feb 15, 2015 · By Marsha Massey. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules. The site includes opportunities to practice filling in electrons, attaching the names/symbols of MOs, and matching orbital overlap drawings to MOs.
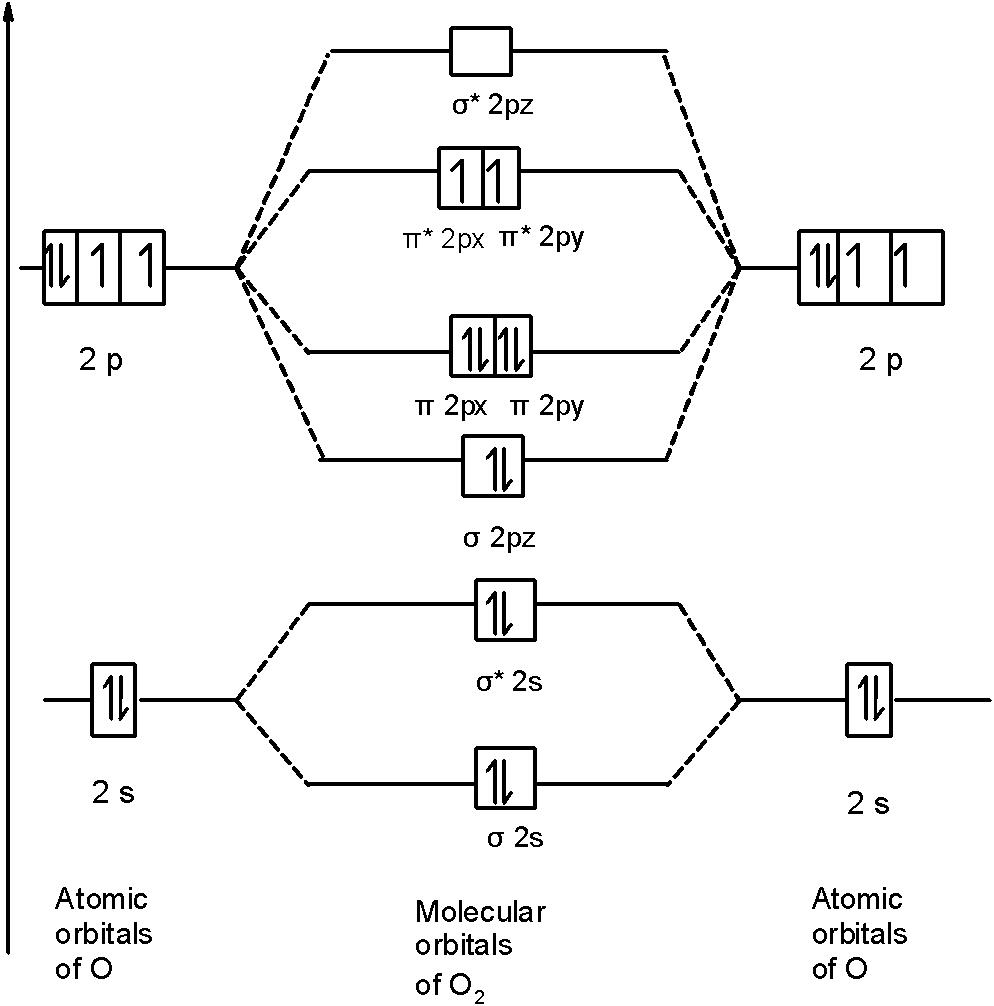
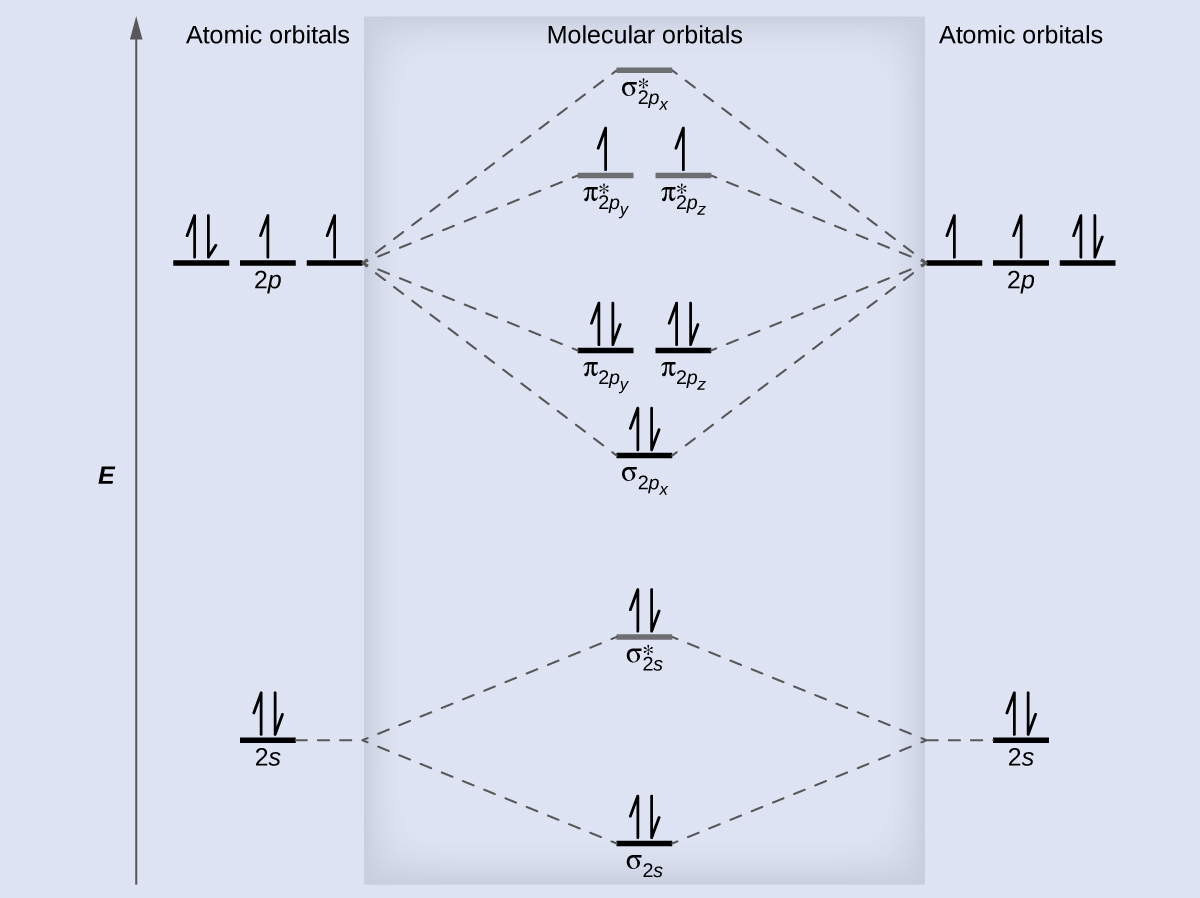
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Practice Problems Molecular Structure and Covalent Bonding. 1. Write Lewis structures for (a) XeF 4, (b) ... Draw a molecular orbital diagram for the hypochlorite ion. What is the bond order? Is this consistent with the bonding predicted by a Lewis dot structure? 17. Draw a molecular orbital diagram for the hydroxide ion.












Comments
Post a Comment