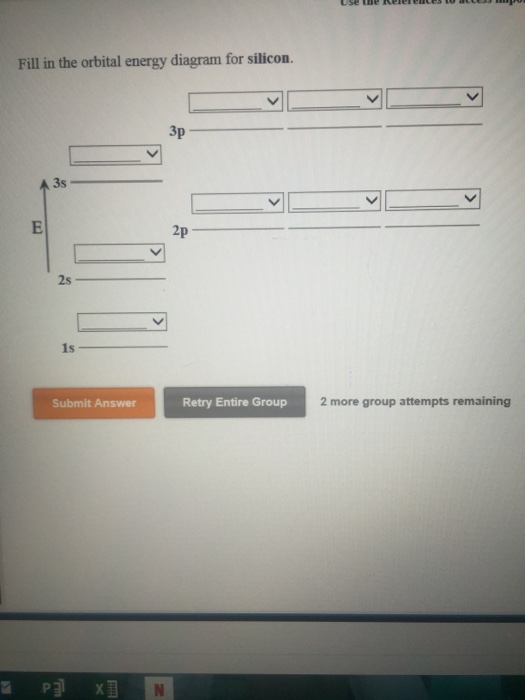
42 silicon orbital diagram
How Many Valence Electrons are in Silicon. There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4. Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. Hydrogen electron configuration is 1s 1.Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.. The first element of the periodic table is hydrogen and its position at the beginning of the periodic table.
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. Orbital diagrams are a pictorial description of electrons in an atom.

Silicon orbital diagram
Question: examine the orbital diagram for the ground state electron configuration of silicon choose best dxplanataion for why this orbital diagram is incorrect foe the ground state of silicon. This problem has been solved! See the answer See the answer See the answer done loading. Thereof, what is the orbital diagram for silicon? Silicon has 14 electrons in the following orbital configuration 1s2 2s2 2p6 3s2 3p2 when neutral in charge. Also the crystalline form is used in semiconductors. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p. In writing the electron configuration for silicon the first two electrons will go in the 1s orbital. 4. Each orbital can hold two electrons. 5. In the second level of a silicon atom (above right), the eight electrons are in a 2s orbital and 3 separate 2p orbitals. 6. A 2p orbital has a shape similar to the diagram on the right. Each electron in a 2p orbital is doing a figure-of-eight loop around the nucleus. 7. At the 1 level, there is only a ...
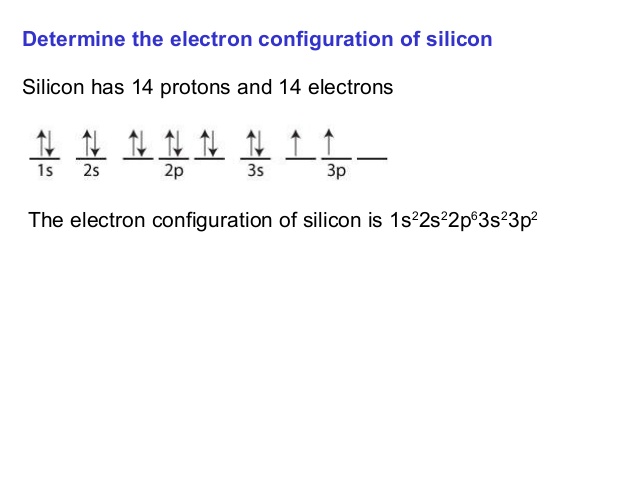
Silicon orbital diagram. Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set of .Which ground-state atom has an electron configuration described by the following orbital diagram? A antimony B germanium C indium D lead E tin%(1). Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom. Silicon contains 14 electrons that are distributed among five energy levels. The orbitals 1s, 2s, 2p and 3s are filled first with 2, 2, 6 and 2 electrons, respectively. The remaining two electrons are placed in the 3p orbital. Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor.It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen ...
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell... The orbital diagram for an element shows the electron distribution of the electrons and the correct pairing of electrons with respect to electron spin. Orbital diagrams are pictorial descriptions of the electrons in an atom. In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Silicon atomic orbital and chemical bonding information. The p orbital can hold up to six electrons. Show the orbital-filling diagram for N (nitrogen). ... Give the ground-state electron configuration for silicon (Si) using noble-gas shorthand. [Ne]3s^23p^2. Item 3: Part C Give the actual ground-state electron configuration for copper (Cu) using the complete form. 1s^22s^22p^63s^23p^63d^104s^1. In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
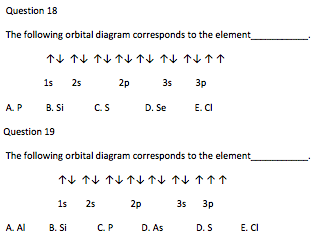
Jun 2, 2018 — All orbitals hold two electrons, and there is one possible orbital for s electrons to have, so we add two electrons to 1s. The same is true ...2 answers · 1s22s22p63s23p2 Explanation: When adding electrons, the lowest energy levels are always ... E. A 1s orbital can be represented as a two-dimensional circle centered around the nucleus of an atom. 3. When completing the orbital diagram for the element silicon, which of the following statements is correct? A. There are no electrons in the 3d sublevel. B. There are no electrons in the 3s sublevel. C. The 2p sublevel is not full. D. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin.
But, the silicon atom forms four sigma bonds as per the Lewis structure of silicon tetrafluoride and VSEPR theory. Hence, one of the electrons from the 3s orbital excite to the 3p orbital of the silicon atom and provide four unpaired electrons for the formation of four sigma bonds with four fluorine atoms.

860-880 North Lake Shore Drive, Electrical Riser Diagram (11/28/1949) // Ludwig Mies van der Rohe (American, born Germany, 1886–1969) Associate Architect: Holsman, Holsman, Klekamp and Taylor (American, 20th century) Associate Architect: Pace Associates (American, 20th century) Structural Engineer: Frank J. Kornacker (American, active 1940s–1950s)
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +½).
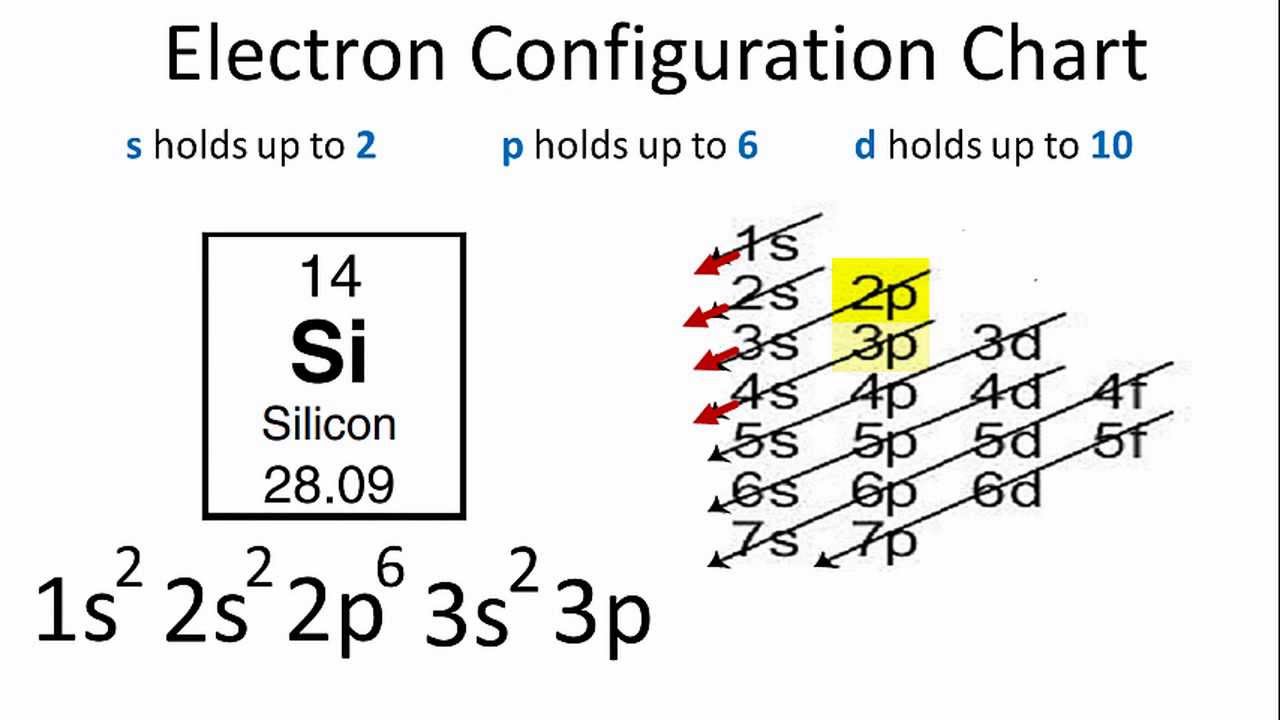
Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2.The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
What is the orbital notation for silicon? Its hard to put this on a screen with a regular key board. But the 1's at the top represent arrows in the pared up arrows imagine the first one pointing ...
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.
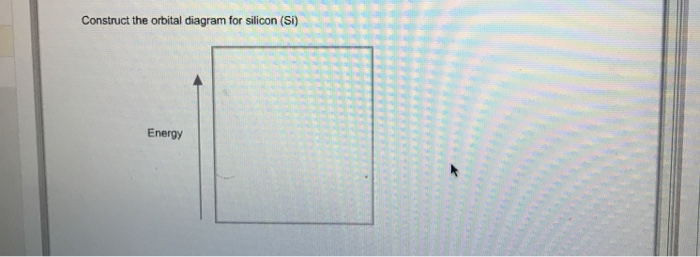
To write the orbital diagram for the Silicon atom (Si) first we need to write the electron configuration for just Si. To do that we need to find the number ...
Given: Silicon atom. An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way. Silicon [N e] 3 s ² 3 p ²
Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair.

Mobile Delousing Unit, Truck Equipment, Plan of Operation and Erection Procedure, Presentation Drawing (1943) // Bertrand Goldberg American, 1913-1997
Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set of . schematron.org! Germanium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4.
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In accordance with the Auf Bau Precept, every electron occupies the bottom power orbital. You leap up somewhat bit in power and we get the 2s orbital that make it the 2p sublevel.
4. Each orbital can hold two electrons. 5. In the second level of a silicon atom (above right), the eight electrons are in a 2s orbital and 3 separate 2p orbitals. 6. A 2p orbital has a shape similar to the diagram on the right. Each electron in a 2p orbital is doing a figure-of-eight loop around the nucleus. 7. At the 1 level, there is only a ...
Thereof, what is the orbital diagram for silicon? Silicon has 14 electrons in the following orbital configuration 1s2 2s2 2p6 3s2 3p2 when neutral in charge. Also the crystalline form is used in semiconductors. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p. In writing the electron configuration for silicon the first two electrons will go in the 1s orbital.
Question: examine the orbital diagram for the ground state electron configuration of silicon choose best dxplanataion for why this orbital diagram is incorrect foe the ground state of silicon. This problem has been solved! See the answer See the answer See the answer done loading.















Comments
Post a Comment