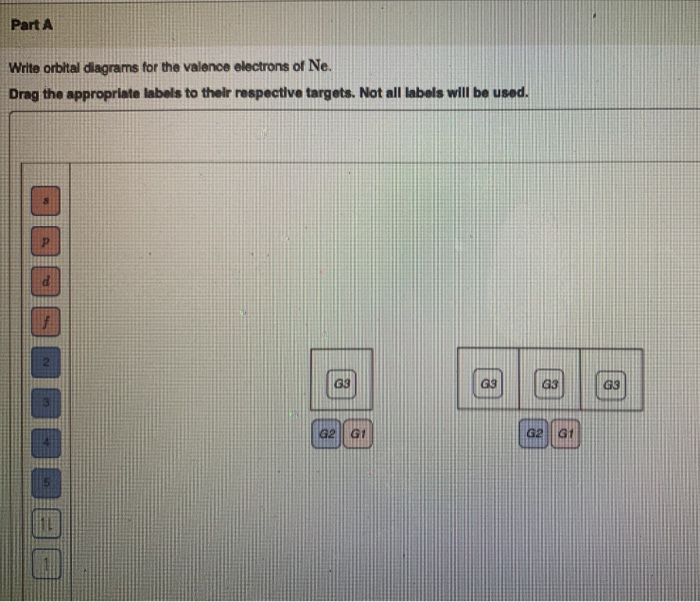
41 orbital diagram for strontium
Electron binding energies for strontium. All values of electron binding energies are given in eV. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules; relative to the Fermi level for metals; and relative to the top of the valence band for semiconductors. Label Orbital eV ... Orbital Diagram For Strontium 09.10.2018 5 Comments In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most.
Each orbital is denoted by three quantum numbers, n, l and m. The number against an orbital is indicated by the energy level of the electron in that orbital. The energy level closest to the nucleus is 1; the next one is 2 and so on. The symbols of the orbital shapes i.e. s, p, d and f come from the words meaning sharp, diffuse, principal and ...
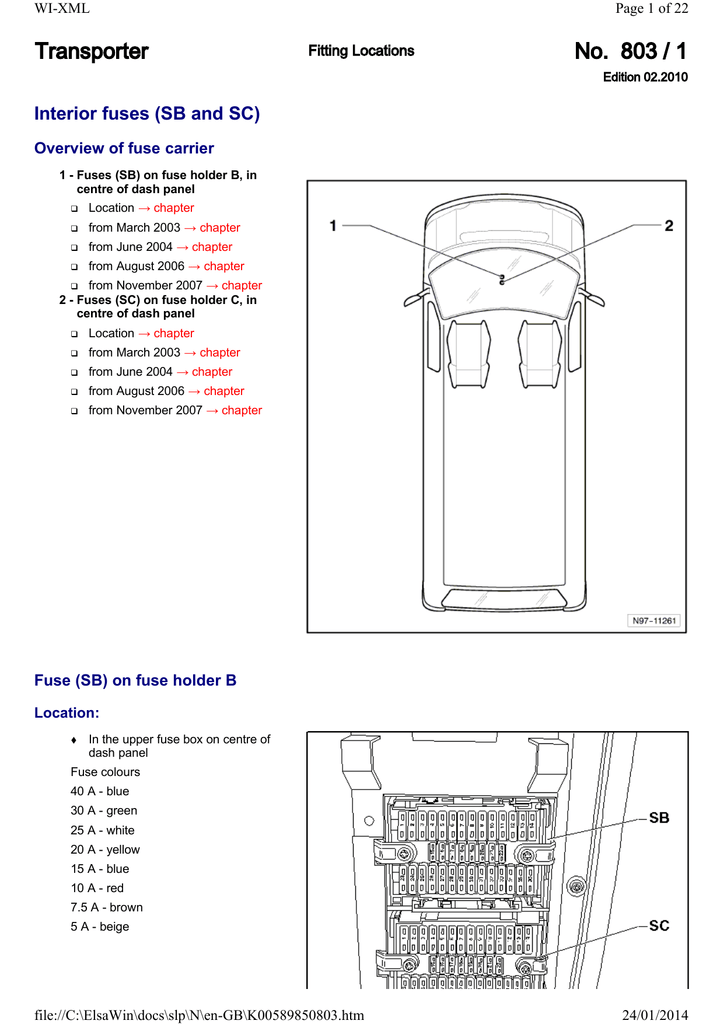
Orbital diagram for strontium
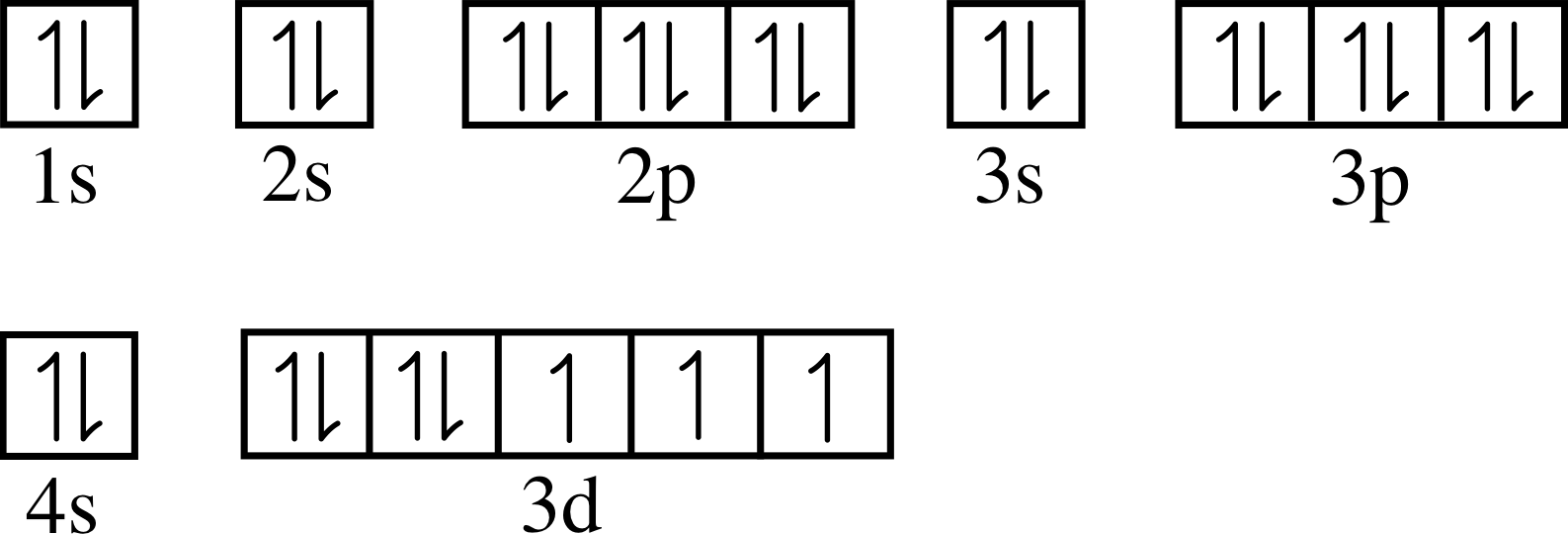
When strontium and chlorine form an ionic compound, strontium, the metal is going to donate its electrons to form a cat eye on and chlorine, a nonmetal is going to accept the electrons to form an an eye on. We can describe this process three different ways. Using electron configurations, orbital notation and electron dot structures, strontium has an electron configuration. Strontium(Sr) is the 38th element in the periodic table and its symbol is ‘Sr’. The electron configuration of strontium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of strontium, compound formation, bond formation have been discussed. Hopefully, after reading this article you will know in ... Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿⇂ ↿⇂ 4d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4f 5s ↿⇂ 5p ↿⇂ ↿ ↿ 5d 5f: ... Strontium atoms have 38 electrons and the shell structure is 2.
Orbital diagram for strontium. orbital diagrams for copper shows that the 3 d orbitals in the experimental diagram are completely filled, whereas, in the theoretical diagram they are not. Therefore, completely filling the 3 d orbitals must confer stability on the electron configuration more than does filling the s orbital. In summary, w hen the 3 d orbitals are either all half- Strontium is atomic number 38 so its electron configuration can be written: 2.8.18.8.2. In orbital notation this is: 1s22s22p63s23p63d104s24p65s2. The noble gas shorthand notation is: [Kr]5s2. When strontium forms a 2+ ion the 2 outer 5s electrons are lost so Sr2+ can be written as: 1s22s22p63s23p63d104s24p6. Electronic configuration of the Strontium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 Electronic configuration of the Strontium atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2 Reduced electronic configuration Sr: [Kr] 5s 2 In order to write the Sr electron configuration we first need to know the number of electrons for the Sr atom (there are 38 electrons). When we write the c...
Strontium Orbital Diagram. In orbital notation this is: 1s22s22p63s23p63ds24p65s2. The noble gas shorthand notation is: [Kr]5s2. When strontium forms a 2+ ion the 2. Comprehensive information for the element Strontium - Sr is provided by this page Comprehensive data on the chemical element Strontium is provided on this. Oxidation States, 2. Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Source: www ... Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of Niobium (Nb) 42: Orbital diagram of Molybdenum (Mo) 43: Orbital diagram of Technetium (Tc) 44: Orbital diagram of Ruthenium (Ru) 45: Orbital diagram of Rhodium (Rh) 46: Orbital Diagram. 1s Soft, malleable, silvery-yellow metal. Uses Used in flares and fireworks for crimson color. Strontium is a long lived highly radioactive fallout product of atomic-bomb. The electron configuration for strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2, according to the Jefferson Lab website.
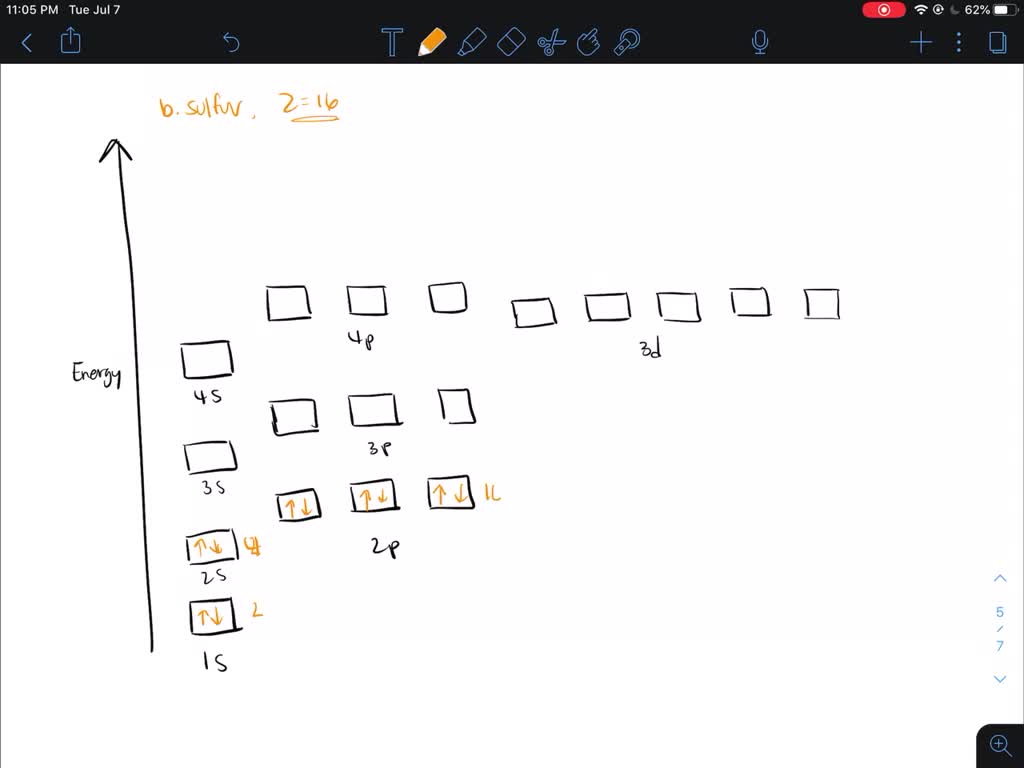
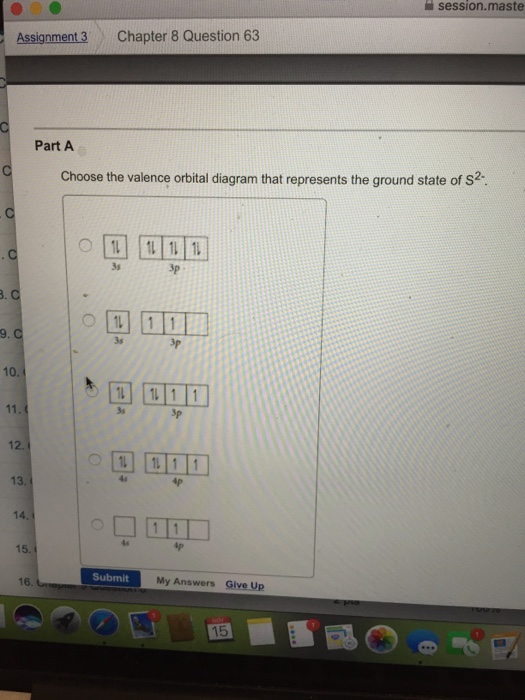
PROBLEM: Use partial orbital diagrams and Lewis symbols to depict the formation of Na+ and O2! ions from the atoms, and determine the formula of the compound. Draw orbital diagrams for the atoms and then move electrons to make filled outer levels. Two sodiums are needed for each oxygen. 3s 3p Na 3s 3p Na 2s 2p O 2s p O2-2 Na+: Na Na + O ... Draw the orbital energy diagram for C to find where its valence electrons are. They are found in 2s,2pₓ,2py,2pz2s,2pₓ,2py,2pz. They are found in 2s,2pₓ,2py,2pz2s,2pₓ,2py,2pz. Which hybrid orbitals overlap in the C - N bond in CO(NH₂)₂? This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment.Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features … Transcribed image text: Electron configuration, orbital diagrams, and Lewis structures of elements and ions A) Write the COMPLETE electrons configuration for strontium (Sr) in its ground state. B) Draw the COMPLETE orbital diagram for Sr in its ground state. C) Write the Lewis symbol for Sr in its ground state. D) Write the electron configuration for Sr as its most state ion.
Orbital Diagram. Strontium Atomic Number And Mass. 1s. ↿⇂. Strontium-90 (90 Sr) is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β − decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and industry and is an isotope of concern in ...
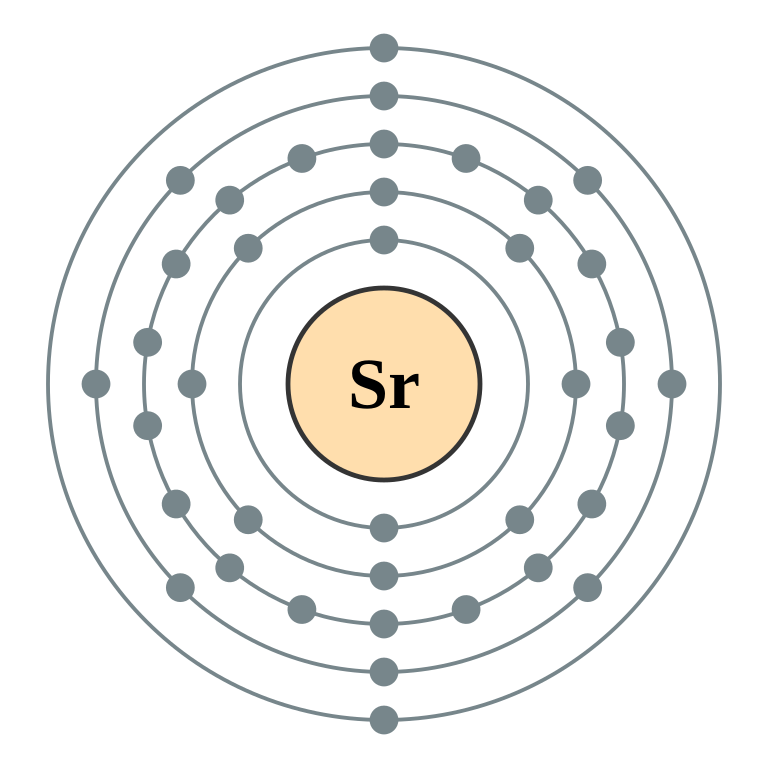
Strontium atoms have 38 electrons and the shell structure is 2.8. 18.8. 2. The ground state electron configuration of ground state gaseous neutral strontium is [Kr]. ... An orbital diagram helps to determine the electron configuration of an element. An element's electron configuration is the arrangement of the electrons in the shells.
Orbitals and Electron Configuration. The Periodic Table with Oxidation Numbers and Electron Configurations. S Block. P Block. D Block. F Block. 1. Hydrogen. 1.
Selenium Orbital Diagram. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4. First, we consider a possibility of a double bond between the two selenium atoms . The corresponding molecular- orbital diagram for the Se 2 dimer, which is a. The orbital diagram is the most detailed picture of showing where all the To ...
Uses of Strontium: Used in flares and fireworks for crimson color. Also used in nuclear batteries in buoys and phosphorescent paint. Additional Notes: A. Crawford first recognized strontium as an element in 1790, but it wasn't isolated until 1808 by Sir Humphry Davy in London England. Strontium Menu. Strontium Page One. Overview of Strontium
Chemistry Q&A Library What is the orbital diagram for the atom Strontium Sr 38. What is the orbital diagram for the atom Strontium Sr 38. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for the atom Strontium Sr 38. check_circle Expert Answer.
Strontium (Sr) Strontium; Orbital Diagram. 1s Soft, malleable, silvery-yellow metal. Uses Used in flares and fireworks for crimson color. Strontium is a long lived highly radioactive fallout product of atomic-bomb explosions. Sources Found in minerals celestite and strontianite.
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
103.95233 (75)#. 105 Sr. 105. 104.95858 (75)#. Mass Number. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of nucleons in an atom. Relative Atomic Mass. The ratio of the average mass per atom of an isotope to 1/12 the mass of a carbon-12 atom.
English: Electron shell diagram for Strontium, the 38th element in the periodic table of elements. Source: Own work: Author: Pumbaa (original work by Greg Robson) SVG development The SVG code is valid. This diagram was created with an unknown SVG tool (generated by script)
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
For Strontium:a) Write the full electron configuration.b) Write the condensed electron configuration.c) Predict the common ion for Strontium.d) Write the con...
Facebook. Twitter. The electron configuration for strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2, according to the Jefferson Lab website. The noble gas configuration of this element is [Kr] 5s2, with [Kr] representing the electron configuration of krypton. Strontium has an atomic number of 38, which means it has 38 protons.

Health Sciences Center, Stony Brook, New York, Sectional Diagram (c. 1974) // Bertrand Goldberg American, 1913-1997
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿⇂ ↿⇂ 4d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4f 5s ↿⇂ 5p ↿⇂ ↿ ↿ 5d 5f: ... Strontium atoms have 38 electrons and the shell structure is 2.
Strontium(Sr) is the 38th element in the periodic table and its symbol is ‘Sr’. The electron configuration of strontium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of strontium, compound formation, bond formation have been discussed. Hopefully, after reading this article you will know in ...
When strontium and chlorine form an ionic compound, strontium, the metal is going to donate its electrons to form a cat eye on and chlorine, a nonmetal is going to accept the electrons to form an an eye on. We can describe this process three different ways. Using electron configurations, orbital notation and electron dot structures, strontium has an electron configuration.

860-880 North Lake Shore Drive, Electrical Riser Diagram (11/28/1949) // Ludwig Mies van der Rohe (American, born Germany, 1886–1969) Associate Architect: Holsman, Holsman, Klekamp and Taylor (American, 20th century) Associate Architect: Pace Associates (American, 20th century) Structural Engineer: Frank J. Kornacker (American, active 1940s–1950s)

Ball Clock (1948/69) // Attributed to Irving Harper for George Nelson Associates American, born 1916 Made by Howard Miller Clock Company Zeeland, Michigan, founded 1926





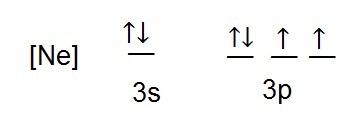















Comments
Post a Comment