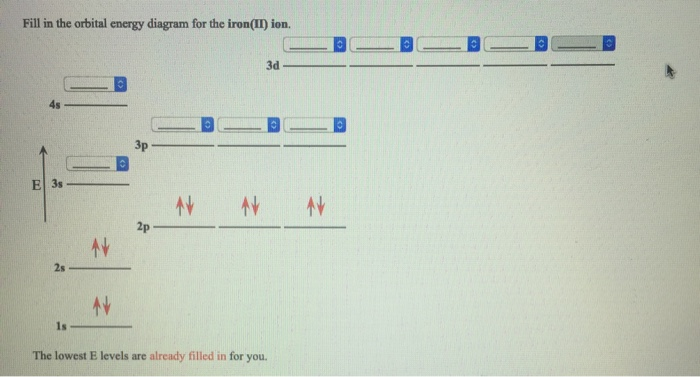
38 orbital diagram of fe
Writing Electron Configurations. The distribution of electrons among the orbitals of an atom is called the electron configuration.The electrons are filled in according to a scheme known as the Aufbau principle ("building-up"), which corresponds (for the most part) to increasing energy of the subshells:. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f Jul 02, 2021 · Schematic illustration of orbital interactions between adsorbed CO (5σ and 2π*) and 3d orbital (d z 2, d xz /d yz) of Fe site in a Fe-SAC and b NiFe-DASC. Differential charge density on c Fe-SAC ...
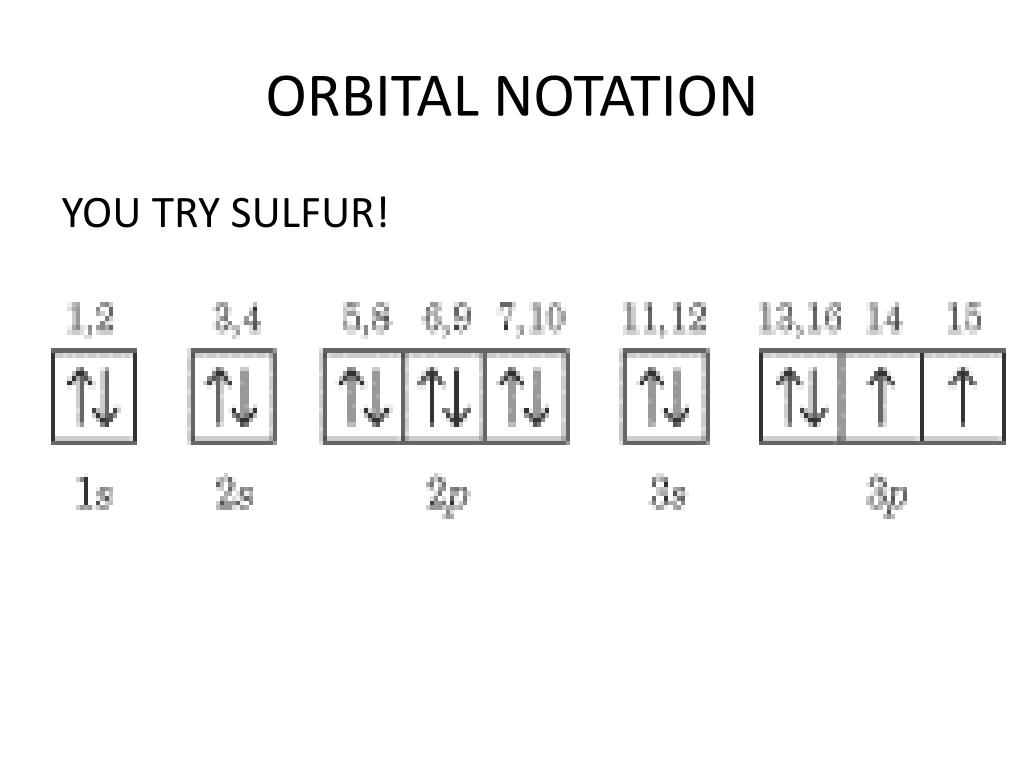
An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
Orbital diagram of fe
01-01-2022 · Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Electronic configuration of the Iron atom. Valence electrons. Orbital diagram. B) f orbital Basically, when l = 0 the is one orbital and it is called an s-orbital. It can hold 2 electrons total. When l = 1, the orbitals are called p-orbitals and there are 3 of them. Each of the individual p-orbitals can hold 2 electrons each. This gives us a total of 6 electrons that can go into the 3 p-orbitals.
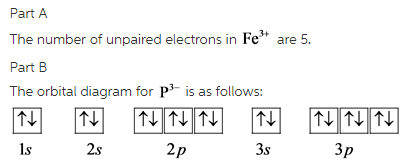
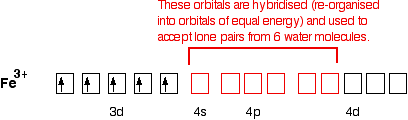
Orbital diagram of fe. Orbital diagrams are a simple way of showing the way the electrons are arranged within an atom or molecule. They are constructed by sequential orbitals from low ...1 answer · Top answer: Iron orbital diagram Iron is in the d-block in the fourth period of the periodic table. This means that the valence electrons are in the 3d... Low-spin [Fe(NO 2) 6] 3− crystal field diagram The Δ splitting of the d orbitals plays an important role in the electron spin state of a coordination complex. Three factors affect Δ: the period (row in periodic table) of the metal ion, the charge of the metal ion, and the field strength of the complex's ligands as described by the ... Site Design: Alan B. Chamberlin URS Clearance: CL#21-4165 CL#21-4165 The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.
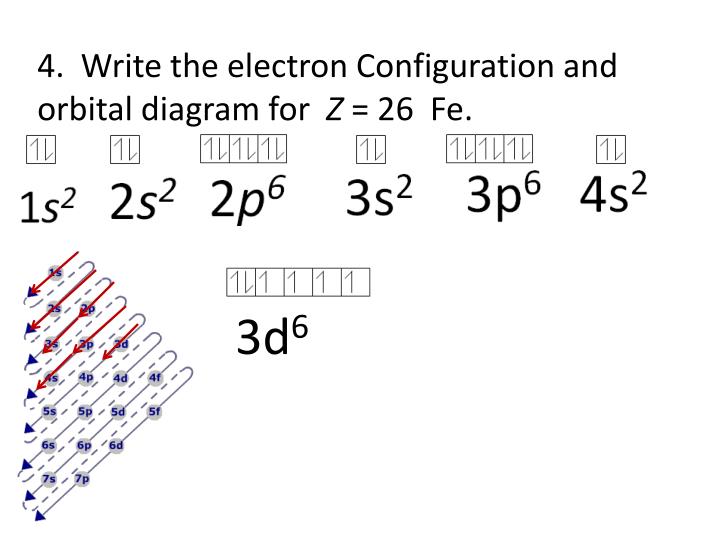
31-08-2021 · Discover what a phase diagram is and how it graphs a substance's states of matter using temperature and pressure. Learn how to define and locate phase equilibrium lines, triple points, critical ... The atomic number of nitrogen is 7 and its symbol is ‘N’. The standard atomic mass of nitrogen is 14.006. The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article. Oxidation States, +3, 2. Electrons Per Shell, 2 8 14 2. Electron Configuration, [Ar] 4s2 3d6. 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Orbital Diagram. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...5 Jul 2019 · Uploaded by Wayne Breslyn
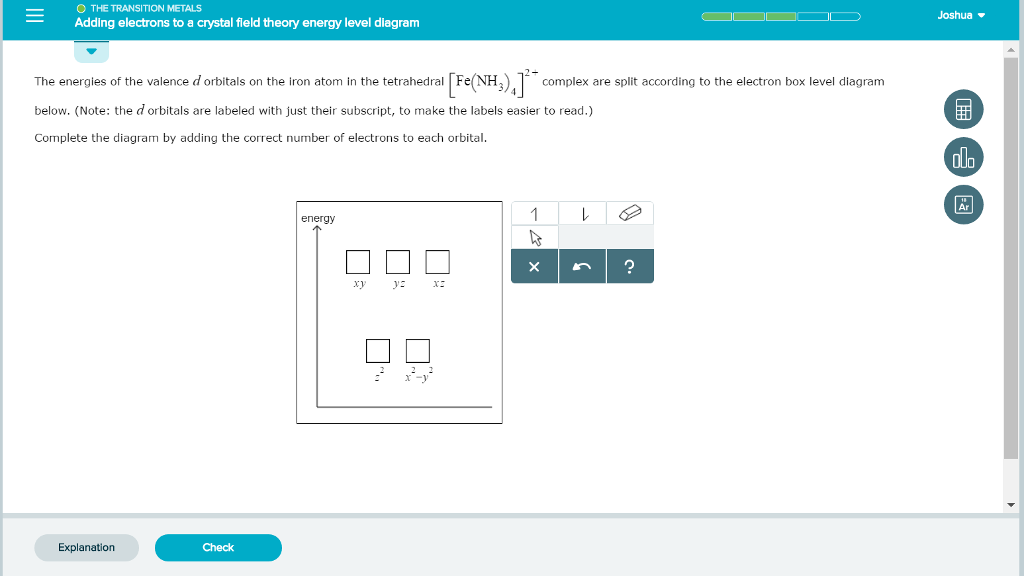
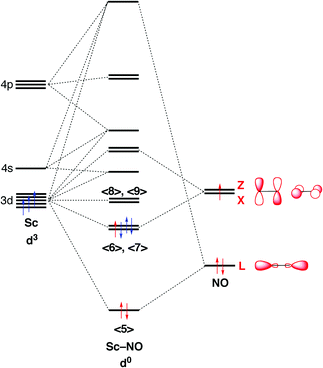
31 Mar 2018 · 1 answerIron has the configuration 1s22s22p63s23p63d64s2. Explanation: Electron configurations of elements can be a bit confusing, and the ... Download scientific diagram | Schematic molecular orbital diagrams for high-spin ( 6 A 1g ) Fe 3+ and [FeCl 6 ] 3− , as well as low-spin ( 2 T 2g ) [Fe(CN) ... For example, in the MO diagram provided for the [Ti(H 2 O) 6] 3+ the ns orbital – which is placed above (n − 1)d in the representation of atomic orbitals (AOs) – is used in a linear combination with the ligand orbitals, forming a very stable bonding orbital with significant ligand character as well as an unoccupied high energy antibonding orbital which is not shown. 29 Jul 2021 — The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to ...
B) f orbital Basically, when l = 0 the is one orbital and it is called an s-orbital. It can hold 2 electrons total. When l = 1, the orbitals are called p-orbitals and there are 3 of them. Each of the individual p-orbitals can hold 2 electrons each. This gives us a total of 6 electrons that can go into the 3 p-orbitals.
Electronic configuration of the Iron atom. Valence electrons. Orbital diagram.
01-01-2022 · Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below.




complexes.png?revision=1&size=bestfit&width=545&height=372)








![Electron orbitals [2010-12-15] | Electrons, Awkward, Rearrange](https://i.pinimg.com/originals/89/ab/8f/89ab8fae9b13b38e354593c867394935.png)








Comments
Post a Comment