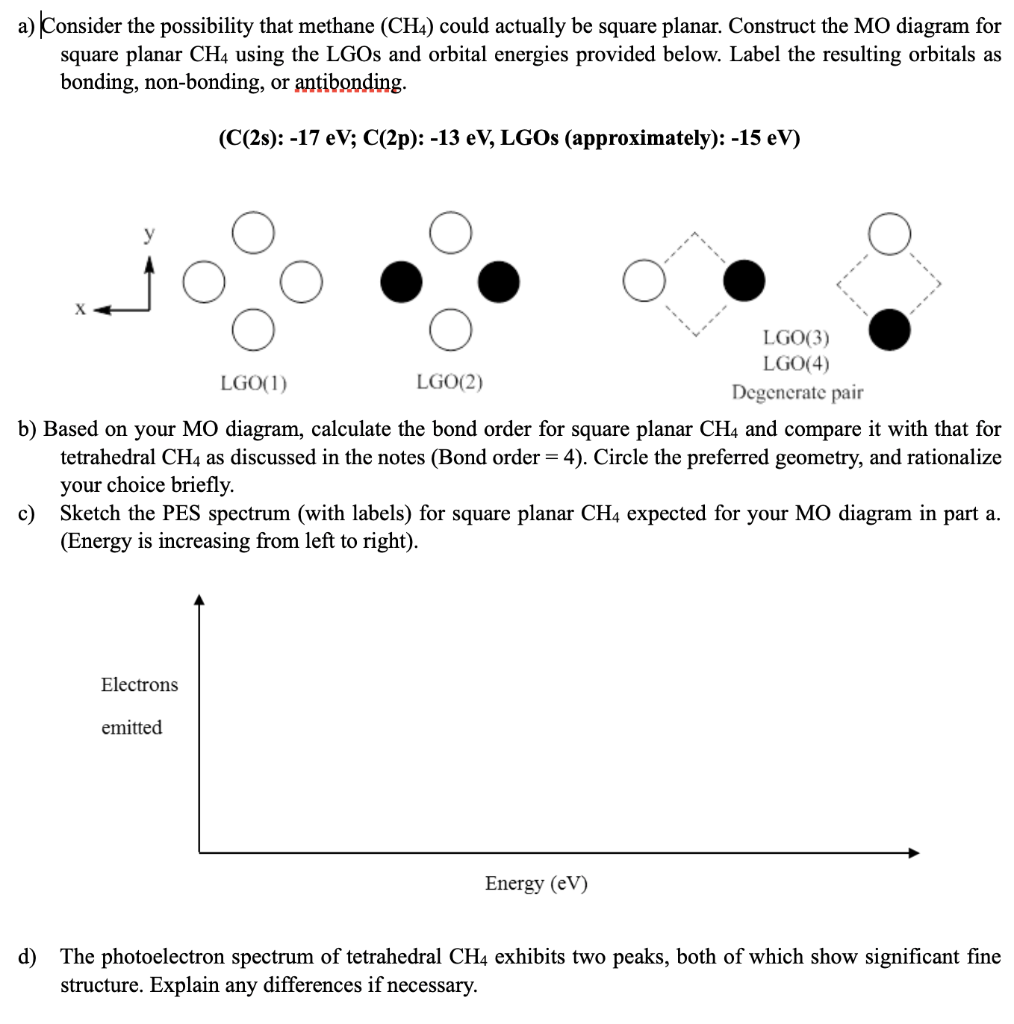
38 methane mo diagram
Methane (CH4) molecular orbital diagram (sigma bonding and antibonding orbitals shown). Firefly 8.2.0 DFT B3LYP 6-311G(d,p) energy minimum (E = -40.5343 Hartrees), Td point group symmetry. MO 2 = -18.94 (1a1); 3-5 = -10.75 (1t2); 6 = 1.41 (2a1) and 7-9 = 3.38 eV (2t2). Note that the lowest energy MO is evenly distributed around the molecule: these two electrons are equally shared between ... Methane energy level diagram
The magnetic method is the most promising method that can be used to inspect large areas of reinforced concrete (RC) structures. Magnetization is a crucial process in this method. The paper aims to present the impact of the magnetization method on the results in the detection of reinforced bars (rebars) and the evaluation of concrete cover thickness in reinforced concrete (RC) structures.

Methane mo diagram
Renewable natural gas (RNG)* is a term used to describe. biogas Gas resulting from the decomposition of organic matter under anaerobic conditions. The principal constituents are methane and carbon dioxide. that has been upgraded for use in place of fossil natural gas. The biogas used to produce RNG comes from a variety of sources, including ... Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. I am having trouble with a question about a lewis dot diagram. 18/10/2020 · Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
Methane mo diagram. best high street shop or best online ... games consoles apple iphone google combined science OCR GCSE Gateway Science chemistry A revision notes high end mobile phones cell phone bargain smartphone xiaomi oppo high tech products combined science OCR GCSE Twenty First Century Science ... November 5, 2021 - The Global Monitoring Laboratory conducts research on greenhouse gas and carbon cycle feedbacks, changes in clouds, aerosols, and surface radiation, and recovery of stratospheric ozone. Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean ... Software Context Diagram. People Also Search ... Reaction. Methane Molecular Orbital. NH4Cl Structure. Nh4 Shape. Nh4 And CL. Nh4 Structure. NH3 Electron Geometry. Ammonium Thiocyanate. Gallery of Nh4 3d. Ralph Lauren Near Me Craigslist Columbia Mo Pets Golf Carts Near Me Craigslist St Louis Cars Craigslist Kansas City Missouri Jeep Dealership ...
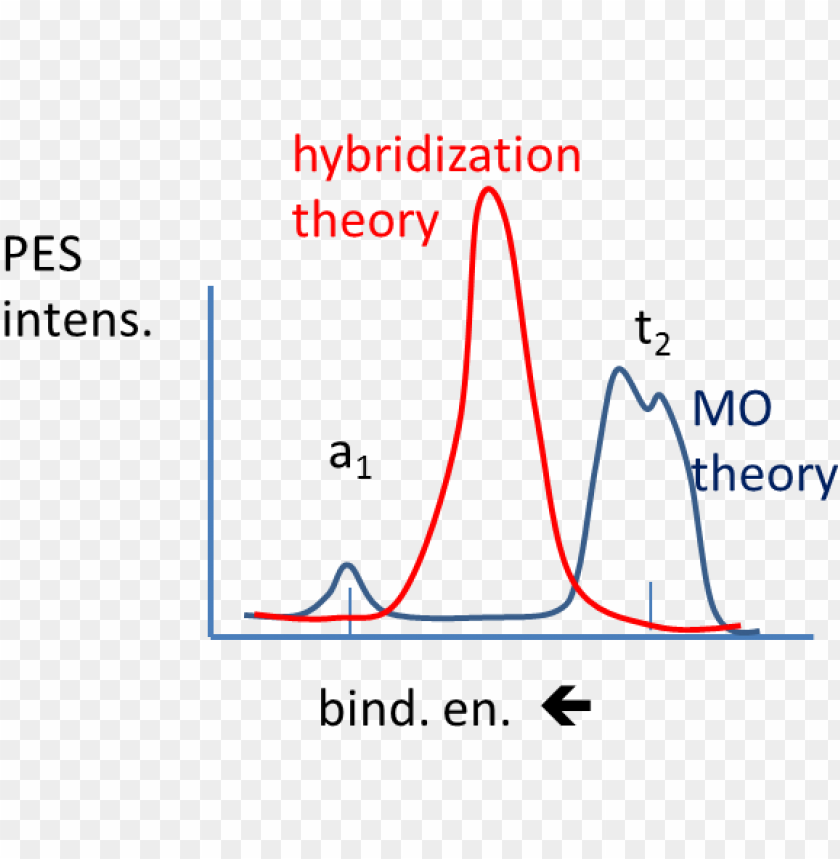
Molecular orbital diagrams, or OM diagrams, are a qualitative descriptive tool that explains chemical bonds in molecules in terms of general molecular orbital theory and linear combinations of atomic orbitals (LCAO) in particular. ... such as methane. OM diagrams can explain why some molecules exist and others don't. They can also predict the ... Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. The molecular formula of methane is C H 4. It is a group- 14 hydride and the simplest alkane. It is a one-carbon compound in which single bonds attach the carbon to four hydrogen atoms. Molar Mass The molar mass of methane, C H 4 = A t o m i c m a s s o f c a r b o n + 4 ( A t o m i c m a s s o f h y d r o g e n) In order to understand the hybridization of CH 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. The type of hybridization involved with CH4 is sp 3. We will discuss in detail how this hybridization occurs below. Name of the Molecule. Methane. Molecular Formula. CH 4.
Chemical, Physical and Thermal Properties of Methane - CH4. Phase diagram included. Methane, CH4, is a colorless odorless gas. It is also known as marsh gas or methyl hydride. The vapors are lighter than air. Methane is easily ignited. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist . Carbonates are readily decomposed by acids. Carbonate molecular shape. It is the conjugate base of the hydrogencarbonate bicarbonate ion HCO. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. 0 Members and 1 Guest are viewing this topic · Page created in 0.107 seconds with 20 queries Methane Molecular Orbitals. In the following model, the carbon atom is dark gray and the hydrogens are cyan. The hydrogen atoms are arbitrarily numbered. A molecular orbital will be displayed by pressing the appropriate button.The different phases of the molecular orbitals are colored red and blue and are separated by nodal surfaces at which electron density is zero.
July 6, 2017 - Dongliang Jin and Benoit Coasne . Molecular Simulation of the Phase Diagram of Methane Hydrate: Free Energy Calculations, Direct Coexistence Method, and Hyperparallel Tempering. Langmuir 2017, 33 (42) , 11217-11230. https://doi.org/10.1021/acs.langmuir.7b02238
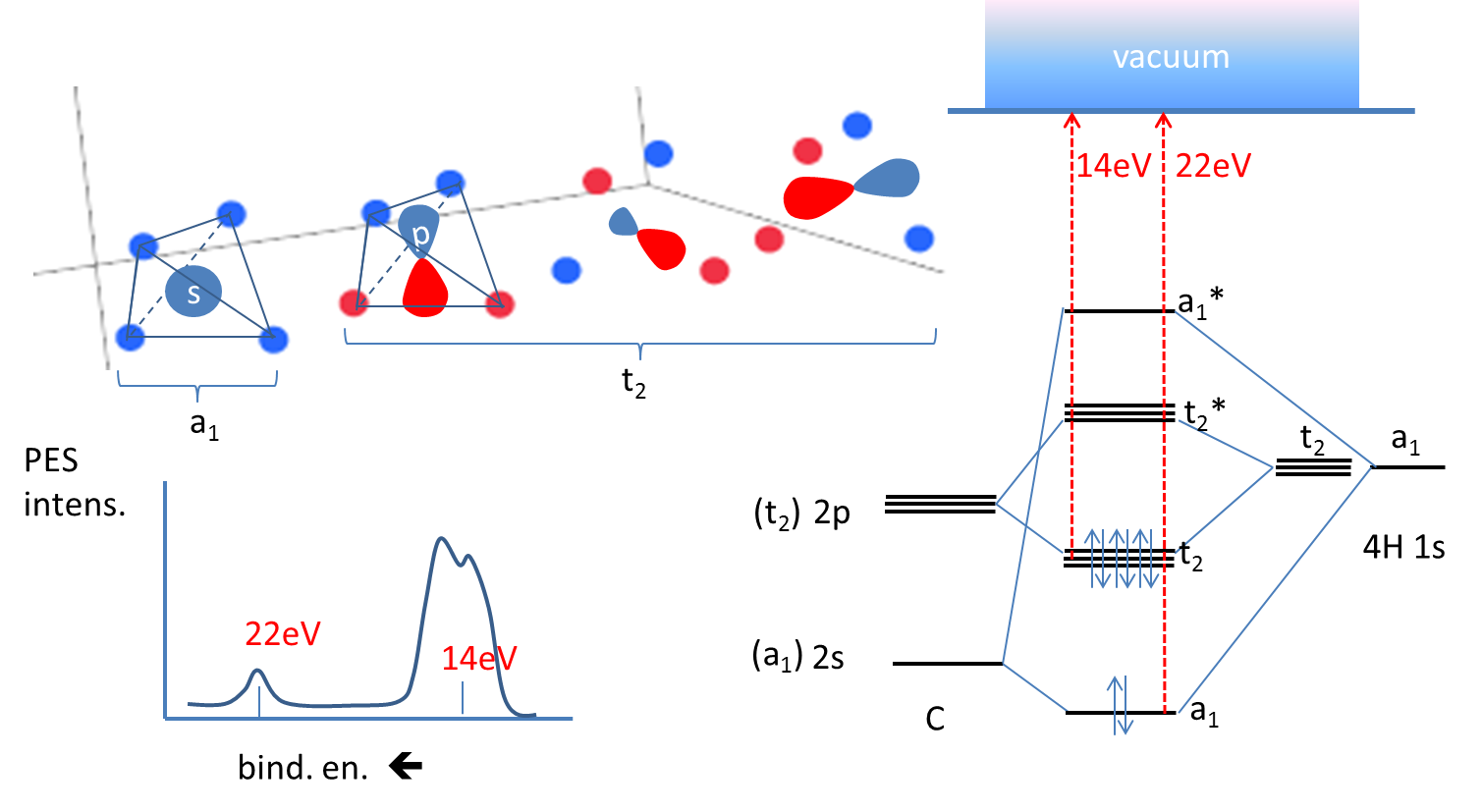
CO2 Molecular Orbital (MO) Diagram. The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
CH4 Lewis Structure, Hybridization, Molecular Geometry, Bond Angle and Shape. Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas.
Description. This guided inquiry activity takes students through the process of constructing an MO diagram for square planar methane. LGOs are constructed using a graphical approach. Students are guided through a process that allows them to use their MO diagram to make a claim about chemical properties. Attachment.
CO2 MOs MO Diagram for CO2 C 2p C 2s bonding MOs antibonding MOs C AOs O LCAOs 1 3 u 3 g 2 u 1 g 1 u 2 u 2 g 1 u 1 g 15. Molecular orbital theory for SF6 molecule- • Electronic configuration of sulphur: • Electronic configuration of Fluorine: • Total number of valence electron: • Hybridization: • Structure of SF6- Feb 3, 2021 — The carbon dioxide MO diagram is based on a C atom and ...
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
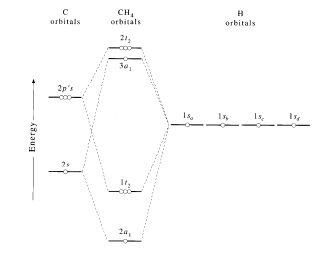
Using LCAO to Construct MOs for Methane
Denitrification experiments of co-combustion of coal and additives were carried out in a horizontal tube furnace. The results showed that calcium acetate limited the production of NO2. The optimum calcination temperature of CTAB-Zr-TiO2 was 673 K. The denitrification efficiency reached up to 72.27%, and desulfurization efficiency reached 83.03% when corncob, calcium acetate, and CTAB-Zr-TiO2 ...
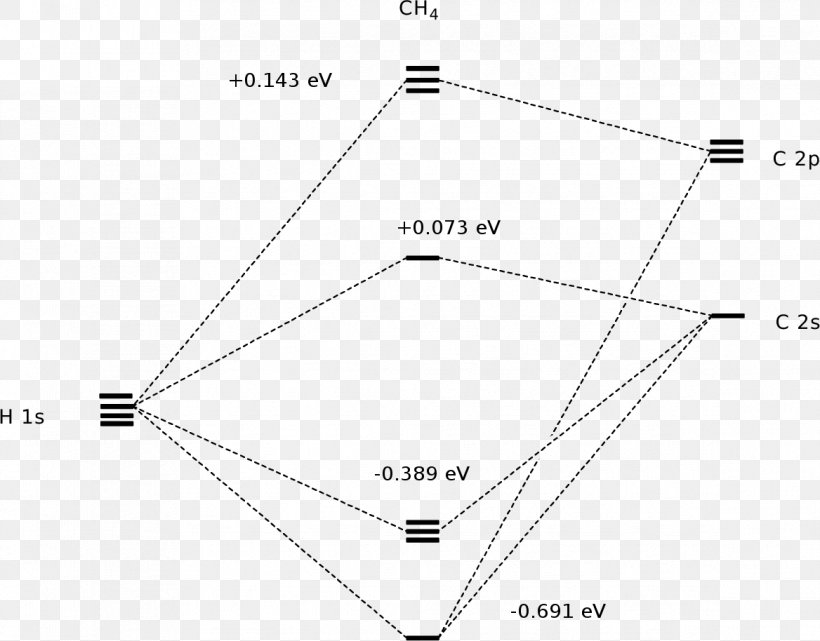
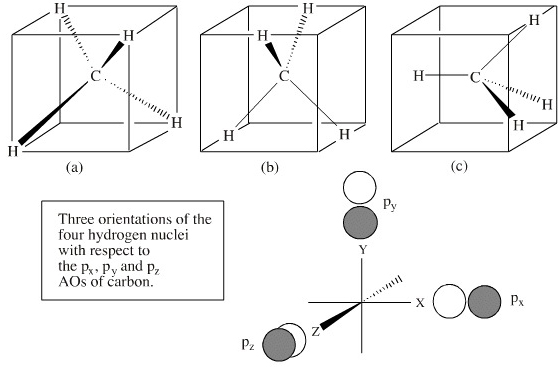
Methane has four valence molecular orbitals (bonding), consisting of one orbital with one nodal plane (lowest occupied) and three degenerate (equal energy) orbitals that do have a nodal plane. For the energy diagram and pictorial view of the orbitals - please see below:
Oxidative coupling of methane (OCM) is a promising technique for converting methane to higher hydrocarbons in a single reactor. Catalytic OCM is known to proceed via both gas-phase and surface chemical reactions. It is essential to first implement an accurate gas-phase model and then to further develop comprehensive homogeneous-heterogeneous OCM reaction networks. In this work, OCM gas-phase ...
This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not.
View flipping ebook version of Skema Jawapan Modul Gemilang A+ Kimia Tingkatan 4 published by faizalnorwen on 2021-08-01. Interested in flipbooks about Skema Jawapan Modul Gemilang A+ Kimia Tingkatan 4? Check more flip ebooks related to Skema Jawapan Modul Gemilang A+ Kimia Tingkatan 4 of faizalnorwen. Share Skema Jawapan Modul Gemilang A+ Kimia Tingkatan 4 everywhere for free.
See how Methane levels have never been higher with this fully interactive Atmospheric Methane (CH4) graph featuring current & historical CH4 levels and global temperatures. A project by the 2 Degrees Institute.
1. Begin with the Lewis Molecular Orbital of Methane, CH4. 1. The Lewis. Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
The merging of four methine spars provides for the construction of methane (CH 4, Fig. 5); likewise, three N-H units plus a nonbonding electron pair lead to ammonia (NH) and a pair of O-H spars and two nonbonding electron pairs are merged to form a C/T structure of water (H 2 O). See the online appendix for further elaboration.
A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
August 15, 2020 - Molecular orbital theory (MOT) for methane forms bonding molecular orbitals involving linear combinations of the unhybridized carbon 2s and 2p valence orbitals with the hydrogen 1s orbitals as shown in the graphic below. The plus and minus signs signify phase, not electrical charge. A molecular orbital diagram ...
MO diagram of homonuclear diatomic molecules ... 4) MO theory and molecular geometry (Walsh diagrams) ... Molecular Orbital Theory – Methane Td.
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons.
Methane is an explosive gas in coalmines and needs to be monitored by methane sensors. Conductive-type methane sensors are small, simple and stable, and they are very promising for mining safety or home safety applications. They can even be employed in mining Internet of things if the power consumption can be lowered down to few milliwatts. Many researches of nanomaterials-based conductive ...
Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms.It is a colourless, odourless, non-toxic but flammable gas (b.p. -161℃). It has a role as a fossil fuel, a member of greenhouse gas and a bacterial metabolite.
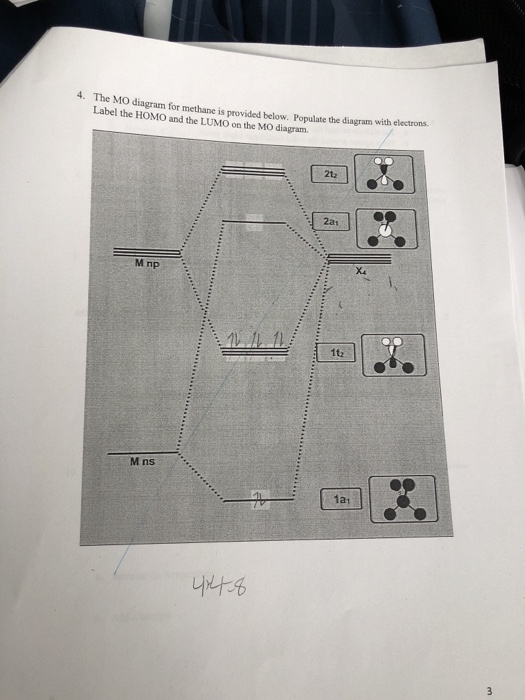
Now we can form the MO diagram for D 4h CH 4 using the carbon AOs and the four LGOs. The instructions are on this page; draw your MO diagram on the blank page that follows. I name the MO diagram and its constituents Name the central atom on one side (the left) and the LGOs on the other (right). Then in the center, place the name of the molecule.
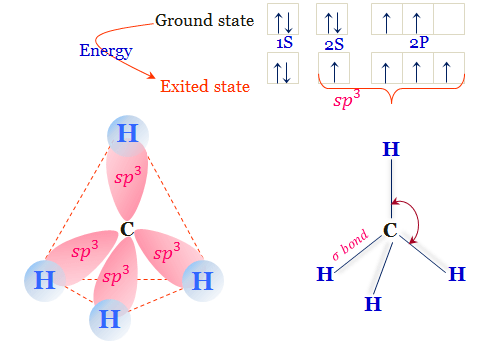
An explanation of the bonding in methane and ethane, including a simple view of hybridisation
In methane, this is achieved at an angle of 109.5°. The 3D structure of the methane is depicted in the below diagram. In the above diagram, carbon is at the center of the structure and four hydrogens bonded to four sp3 hybrid orbitals of carbon. The angle between H-C-H is 109.5°.
November 29, 2021 - Tetrahedral geometry of methane: (A) stick-and-ball model and (B) diagram showing bond angles and distances. (Plain bonds represent bonds in the plane of the image; wedge and dashed bonds represent those directed toward and away from the viewer, respectively.)
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Methane is a tetrahedral molecule with four equivalent C–H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with ...
Molecular Orbital (MO) Diagram In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2.
Chemistry. Chemistry questions and answers. 4. The MO diagram for methane is provided below. Populate the diagram with e Label the HOMO and the LUMO on the MO diagram. 2t2 2a1 X4 M np 1t2 M ns 1a1. Question: 4. The MO diagram for methane is provided below. Populate the diagram with e Label the HOMO and the LUMO on the MO diagram. 2t2 2a1 X4 M ...
Methane | CH4 | CID 297 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
The effect of laser welding on the mechanical properties and the prediction of formability for austenitic stainless steel AISI 304 and ferritic steel AISI 430 when welded by a YLS-5000 fiber laser, were studied in the paper. The microstructure of the welded joint was analyzed using light microscopy. The mechanical properties were determined by static tensile testing. The forming limit diagrams ...
18/10/2020 · Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. I am having trouble with a question about a lewis dot diagram.
Renewable natural gas (RNG)* is a term used to describe. biogas Gas resulting from the decomposition of organic matter under anaerobic conditions. The principal constituents are methane and carbon dioxide. that has been upgraded for use in place of fossil natural gas. The biogas used to produce RNG comes from a variety of sources, including ...




















Comments
Post a Comment