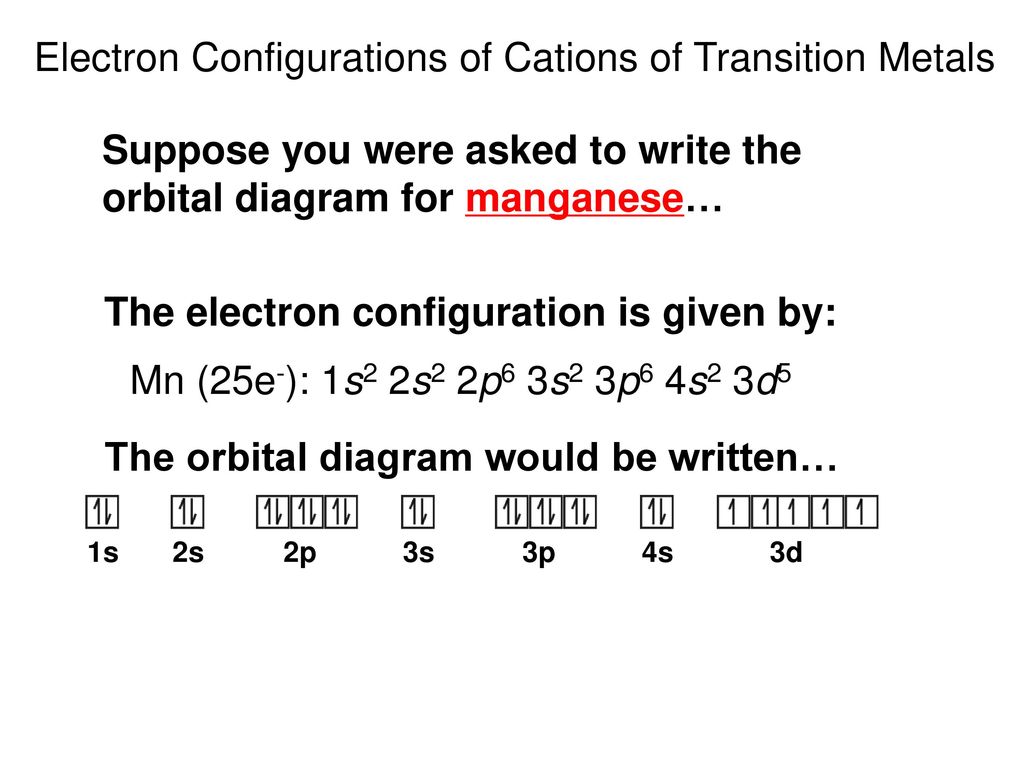
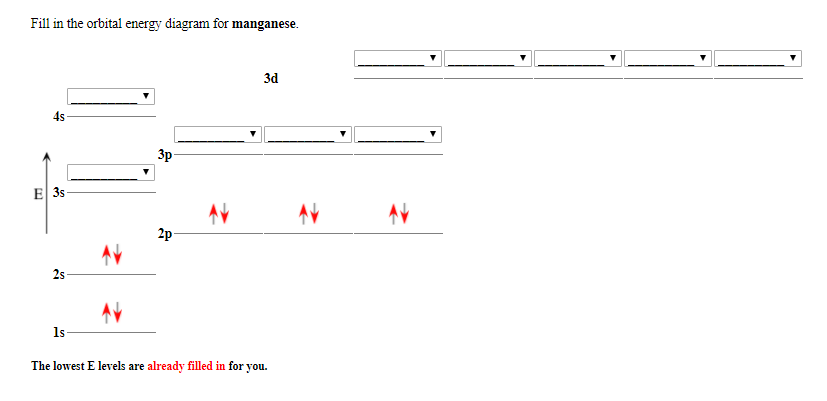
41 manganese orbital diagram
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 is the orbital notation for manganese. Orbital notation requires arrows denoting the spin of each electron. For the purposes of the answer, I'll simply provide ... Orbital Diagram. 1s ... The steel in railroad tracks can contain as much as 1.2% manganese. It is crucial to the effectiveness of vitamin B1. Sources Most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace.
The first and third electron orbital diagrams are possible in the excited state, but not in the ground . state. The second electron orbital diagram (violating Pauli principle) is never possible. 34. Nitrogen: 1s 2 2s 2 2p 3 2s 2p [He] Manganese: ...

Manganese orbital diagram
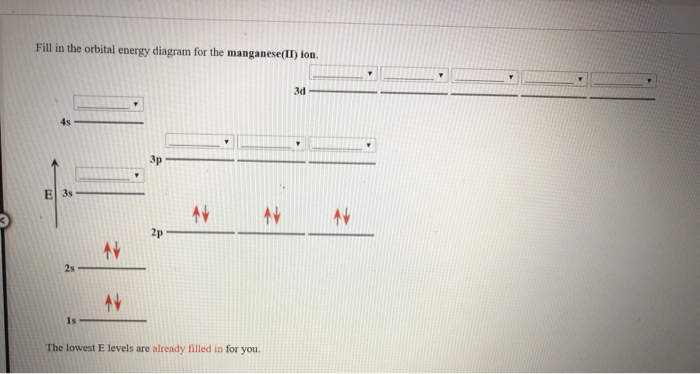
The orbital diagram of {eq}\rm M{n^{8 + }} {/eq} is: c Electron affinity is the tendency to gain electrons, thus if Mn gains 5 electrons it will achieve a charge of -5 and its electronic ... Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what... The placement of the next electron must follow Hund's rule. The orbital diagram shows three unpaired electrons. The electron configuration for nitrogen is 1s 2 2s 2 2p 3. For oxygen the eighth electron must pair with one of the electrons in the 2p orbitals. The orbital diagram for oxygen is shown on the left.
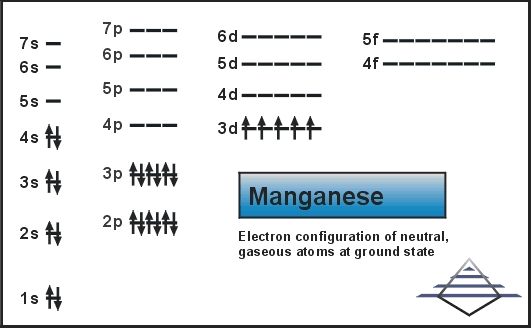
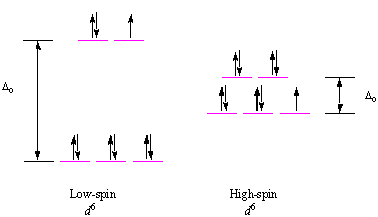
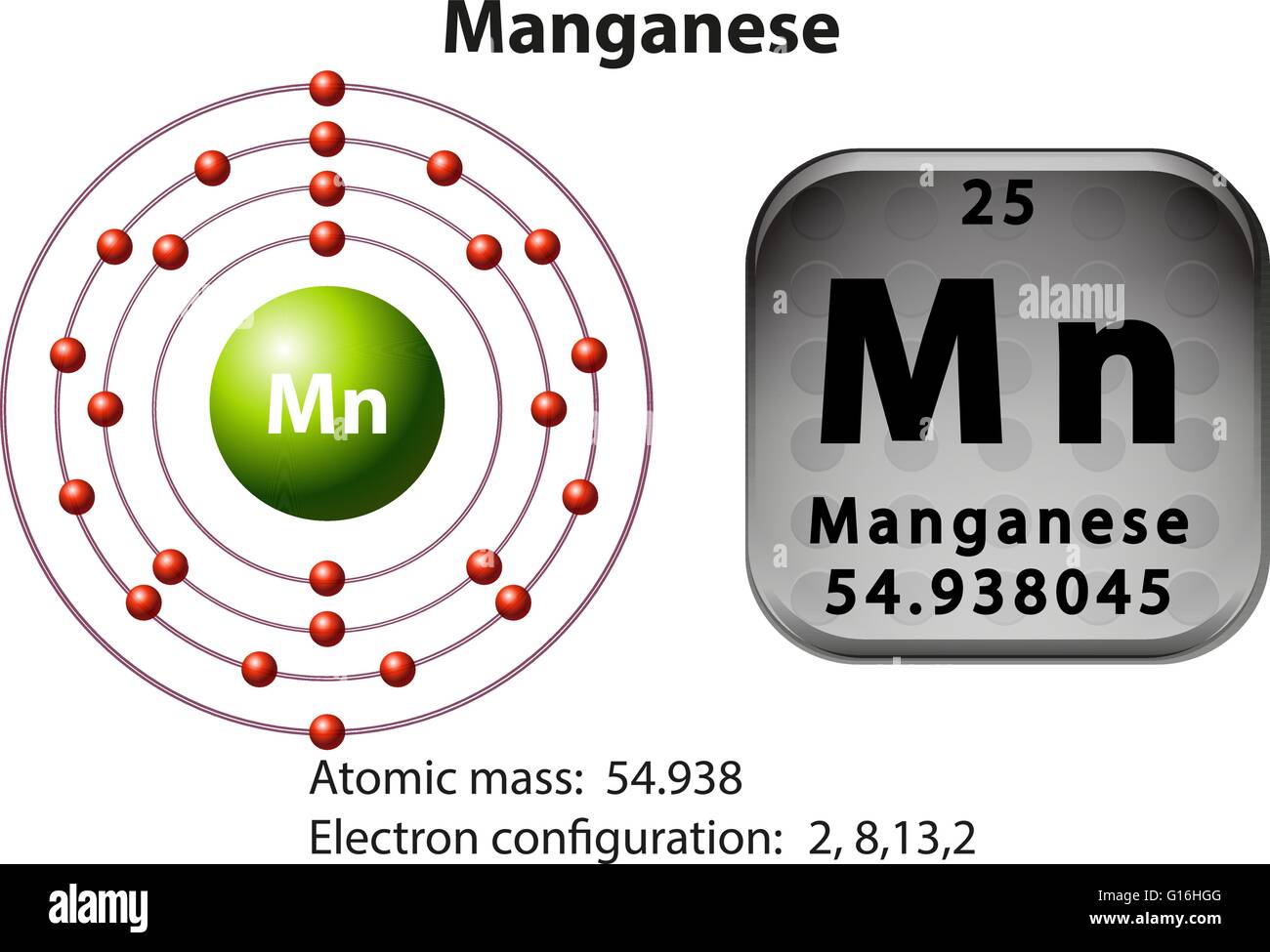
Manganese orbital diagram. Particularly, when two-orbital models are studied, the results are in good agreement with a large list of experimental observations reviewed here. Tendencies toward CI states exist in real manganese oxides all around the FM phase in the temperature-density phase diagram. Orbital-filling Diagram for Abbreviated Electron Configuration of the Metal Ion # of unpaired electrons Magnetism (para or dia) Calcium Sulfate CaSO4 Ca2+ [Ne]3s23p6 0 dia Cobalt (II) Sulfate CoSO4 Co2+ Copper (II) Sulfate CuSO4 Cu2+ Iron (II) Sulfate FeSO4 Fe2+ Magnesium Sulfate MgSO4 Mg2+ Manganese (II) Sulfate MnSO4 Mn2+ Compound properties. Element reactions. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. The ground state electron configuration of ground state gaseous neutral manganese is [ Ar ]. 3d5. 4s2 and the term symbol is 6S5/2. Schematic electronic configuration of manganese. The Kossel shell structure of manganese. Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium ...
Use the orbital-filling diagram to show the electron configuration of helium, He. ... Manganese has 5 electrons in its 3d sublevel. How many electrons are in the 3p sublevel for phosphorous? [∵ 4s orbital has less energy level than 3d orbital, thus electrons will fill 4s before filling the 3d . For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. Figure 2B shows a schematic spin-orbital phase diagram in the moderately doped (0.3 < x < 0.7) manganese oxides on the plane of the uniaxial strain measured as the ratio of lattice parameter c/a (or almost equivalently the ratio of the apical to equatorial Mn-O bond length) and the doping x. Download scientific diagram | Molecular orbital diagram (top) demonstrates the electron transfer process during the metal centered oxidation of Mn(H 2 O) 2+ ...
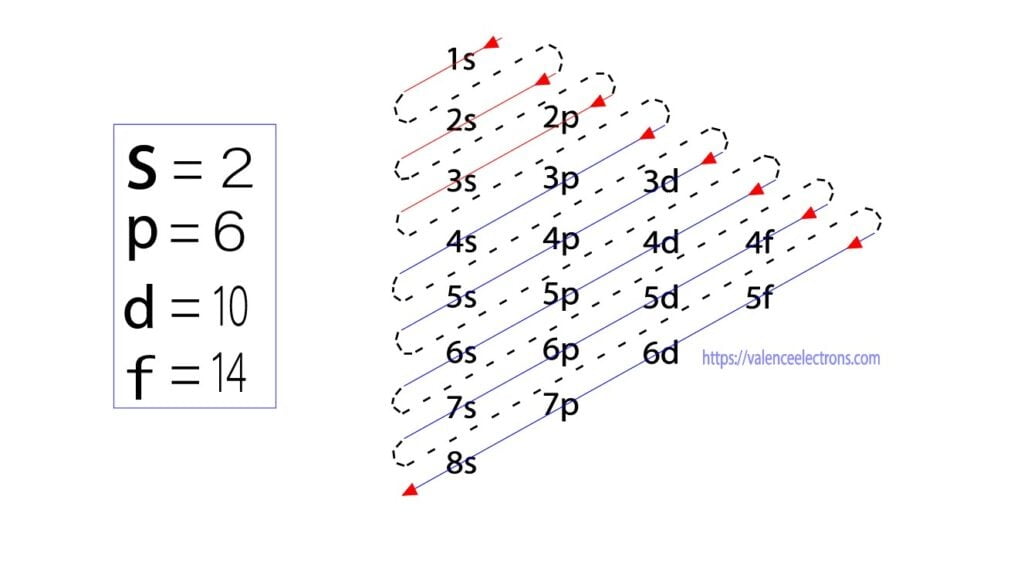
Jul 28, 2021 — Electron Configuration for Mn · With the Aufbau principle, the first orbital has 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on electrons. · Subsequently, ... However, while a small frontier orbital based active space is apparent for MnO 4 À , consisting of the highest occupied oxygen p orbitals and the unoc- cupied manganese d set (partially ... A 3p orbital is smaller than a 2p orbital (T/F) False. A new electron will enter into _______ if 3d orbital is completely filled. 4p orbital. It's impossible for two electrons to occupy the same 2s orbital because it is a violation of the Pauli Exclusion Principle. (T/F) False. The electron configuration. 1s^2 2s^2 2p^6 3s^2 2p^4 corresponds to ... You can determine the ground-state electron configuration of Manganese (Mn) by referring to the periodic table and locating the position of Mn in the periodic table. Ground-state means that the element is in its lowest energy form (not in an excited state). Mn has no charge which means that no electrons are removed or added in the atom. 80% ...
Mn (Manganese) is an element with position number 25 in the periodic table. Located in the IV period. Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5.
Jul 29, 2016 — The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . The diagram below represents the electron configuration as ...1 answer · Refer to the explanation. Explanation: The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents ...
The electron configuration for manganese is 1s2 2s2 2p6 3s2 3p6 4s2 3d5. It can be shortened to [Ar] 4s2 3d5, where the [Ar] represents argon, the last element in the third row of the periodic table, whose electrons fill every shell prior to the 4s-orbital. The first number in each grouping represents the energy level.
adshelp[at]cfa.harvard.edu The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Agreement NNX16AC86A
What is the correct orbital diagram for arsenic? — Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ...
What is the orbital diagram for manganese? Answers: 2 Show answers Another question on Chemistry. Chemistry, 21.06.2019 22:30. Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to ...
• Manganese(Z=25)hasavalenceconfiguration[Ar]4s23d5,andtypicallyshows positive oxidations states of +2, +3, and +7, all of which are seen in this experiment. MnCl 2.4H2O Mn(II) [Ar]3d5 pale pink Mn(acac)3 Mn(III) [Ar]3d4 lustrous dark brown KMnO Mn (VII) [Ar ] deep purple
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the numb...
Chemistry questions and answers. Manganese is found as MnO2 in deep ocean deposits. Choose the electron configuration of this element using the noble gas notation and an orbital box diagram 3d IAfl ↑ ↑ ↑ ↑ ↑ ↑↓ 3d 4s 4s 3d 4s 3d 4s aUsing an orbital box diagram, choose the electron configuration of Pt. 4f 5d 6s 4f 5d 6s 4f 5d 6s ...
May 14, 2018 — So we need to draw the orbital diagrams box and line notation for the ... Okay, so let's go ahead and draw a orbital diagram for manganese ...
manganese mn chemicalaid manganese mn has an atomic mass of 25 find out about its chemical and physical properties states energy electrons oxidation and more. Hf Molecular Orbital Diagram - Orbital Diagram For Fluorine Awesome 0d Mos2 2d G. arrangements of electrons in the orbitals of an atom is the orbital diagram the electron configuration ...

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Manganese: Mn is the chemical symbol of the manganese element. Manganese is a transition element and has a partially filled d-subshell. It belongs to the 3d series of the transition elements.

Solved Draw A Partial Valence Level Orbital Diagram And Write The Condensed Ground State Electron Configuration For Each Begin Array Ll Text A Mathrm Mn Text B Mathrm P Text C Fe End Array
The Latimer diagram for a series of manganese species in acidic solution is shown below. The standard reduction potential for the reduction half-reaction involving the two species joined by the arrow is shown above the arrow. Latimer diagrams show the redox information about a series of species in a very condensed form.
Manganese is a transition metal with a molar mass of 54.94g/mol. Manganese is considered critical for human health, and plays important roles in development, metabolism, and the antioxidant system. That said, excessive manganese intake is associated with manganism, a neurodegenerative disorder that causes dopaminergic neuronal death and parkinsonian-like symptoms.
The placement of the next electron must follow Hund's rule. The orbital diagram shows three unpaired electrons. The electron configuration for nitrogen is 1s 2 2s 2 2p 3. For oxygen the eighth electron must pair with one of the electrons in the 2p orbitals. The orbital diagram for oxygen is shown on the left.
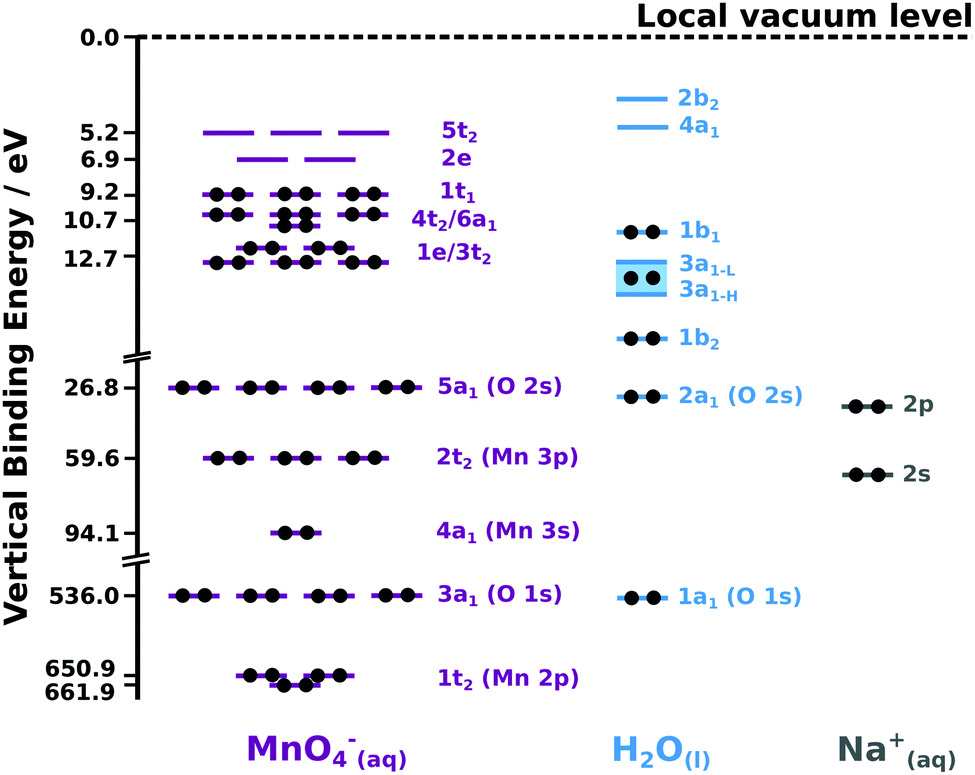
The Electronic Structure Of The Aqueous Permanganate Ion Aqueous Phase Energetics And Molecular Bonding Studied Using Liquid Jet Photoelectron Spectr Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cp04033a
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
The orbital diagram of {eq}\rm M{n^{8 + }} {/eq} is: c Electron affinity is the tendency to gain electrons, thus if Mn gains 5 electrons it will achieve a charge of -5 and its electronic ...

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2

Electron Configurations Activity 16 Intro 3 Rules To Assign E To Orbitals Aufbau E Occupy Lowest Energy Orbital Available Pauli Exclusion Ppt Download

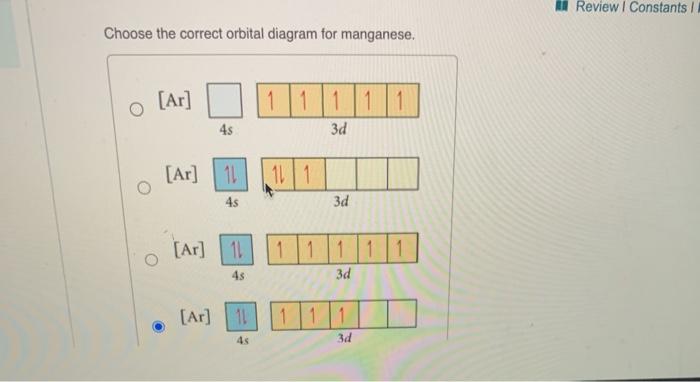
Stion 15 Part A Which Is The Correct Orbital Diagram For Manganese 3d Submit Request Answer Homeworklib


/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)














Comments
Post a Comment