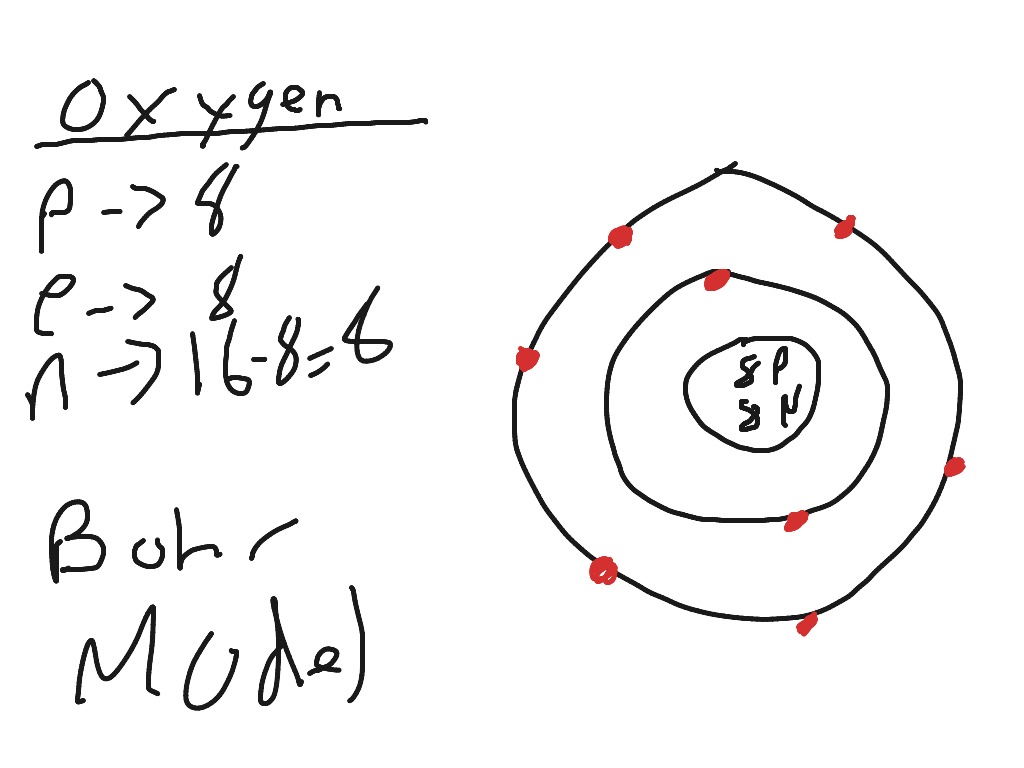
39 mg bohr diagram
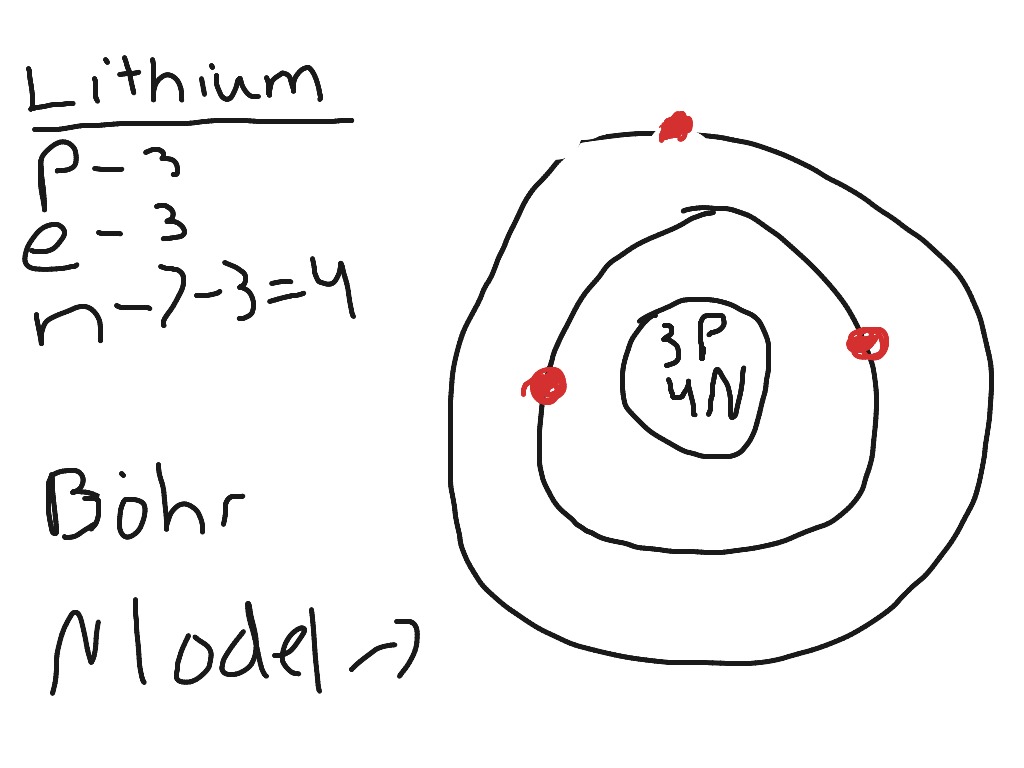
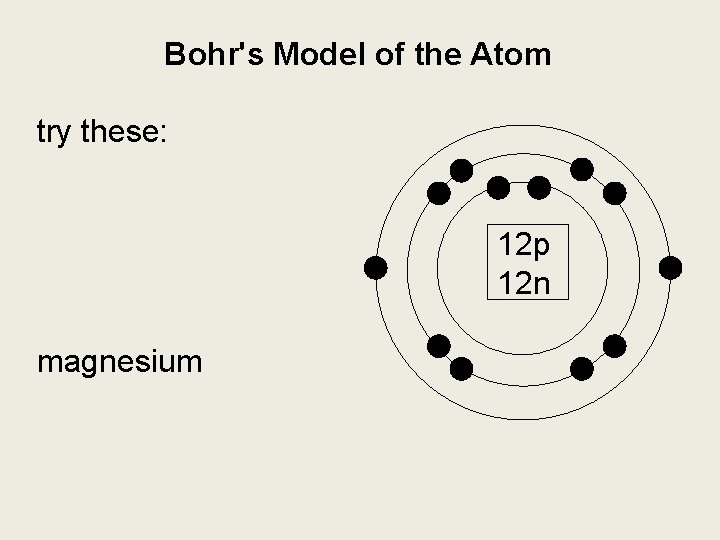
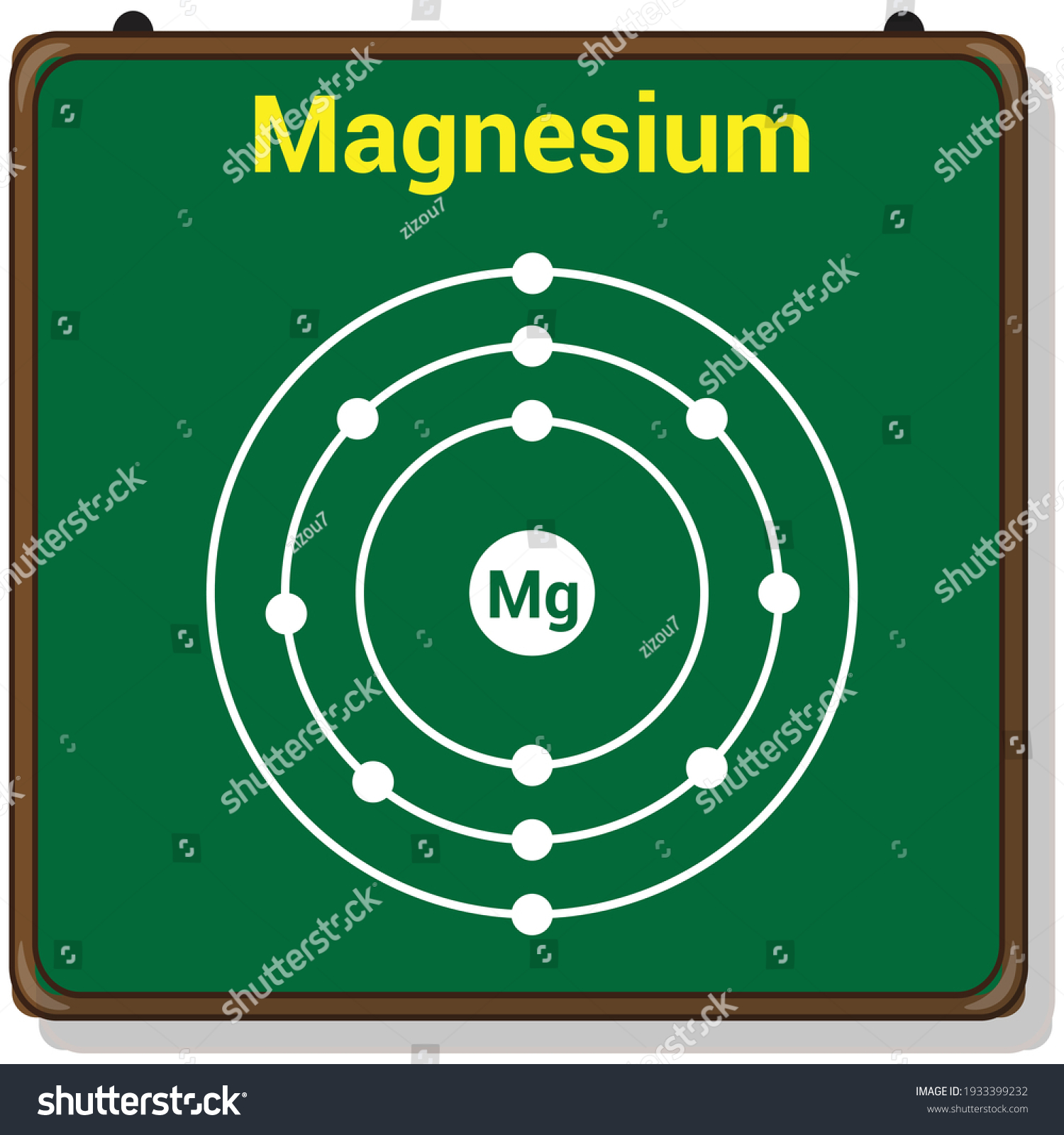
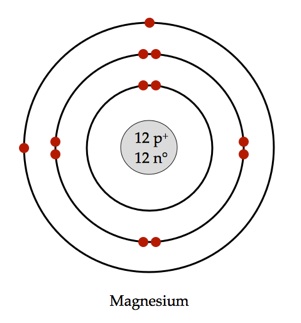
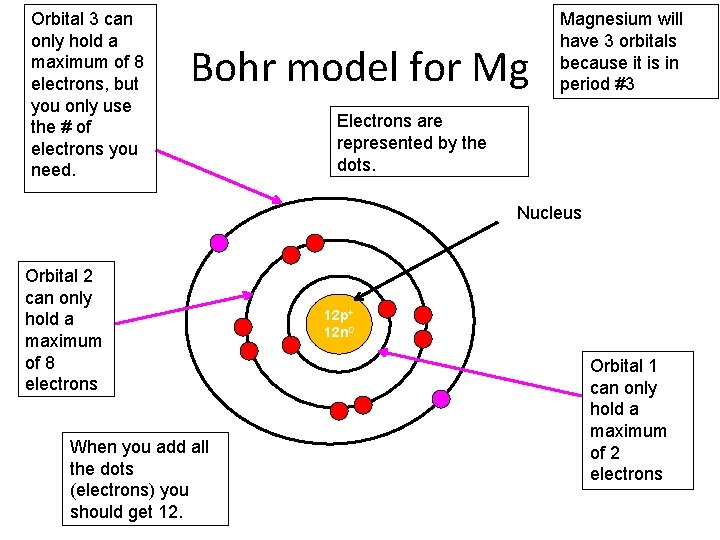
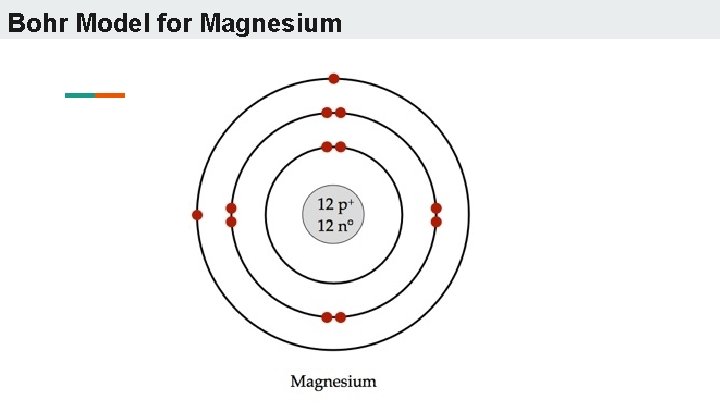
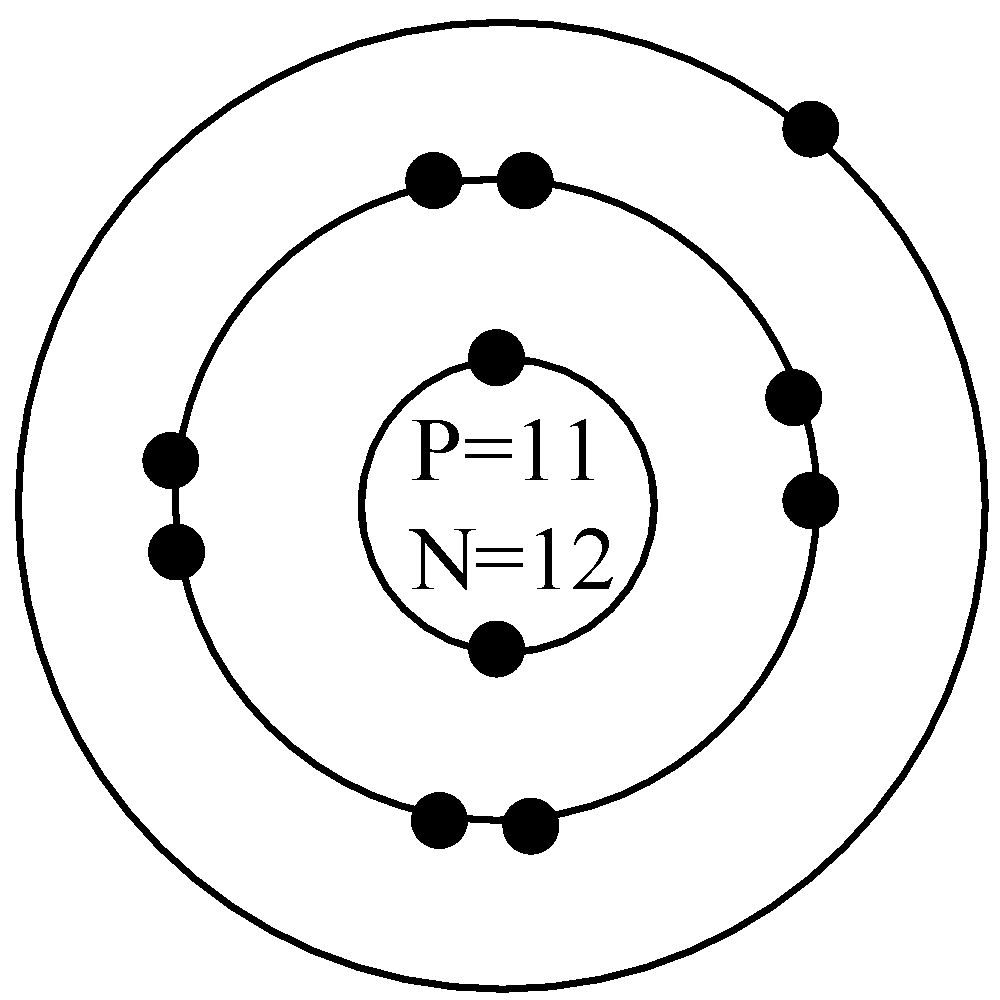
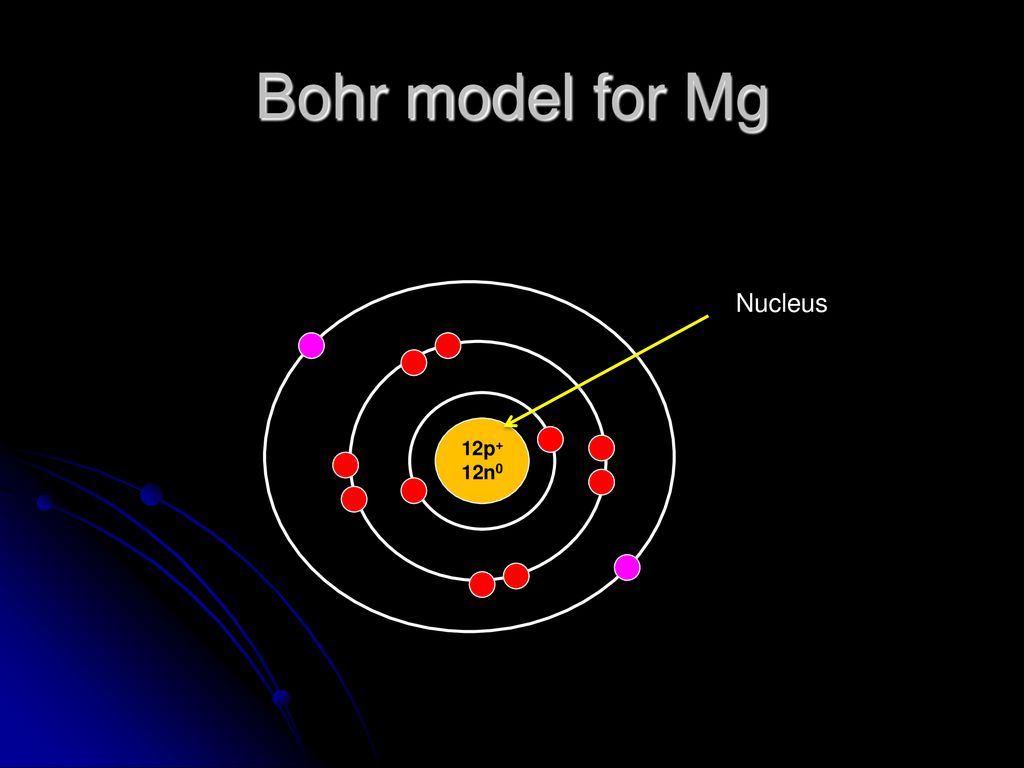
Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Apr 28, 2015. Magnesium has 3 energy levels: 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. In the nucleus you would show the 12 neutrons and 12 protons. Answer link.
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
Mg bohr diagram
Part 1: Bohr Diagrams. 1. Complete the following table by drawing the correct Bohr models for each element. Hydrogen. Lithium. Magnesium. Oxygen. Chlorine. Step 7: Draw a Bohr diagram and Lewis Structure to show the arrangement of electrons and the number of valence electrons. ... Green = Li & Na Pink = O & S Blue = Be & Mg Purple = F & Cl Orange = B & Al Red = C & Si Tan = N & P Yellow = He, Ne, & Ar Step 9: Cut the cards apart and arrange according to atomic number in the pattern shown. Once you Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds two electrons. The second holds 8. So far, 10, of magnesium's 12 ...1 answer · Top answer: Hint :We know that by drawing a nucleus with required number of shells. Since the total number of electrons in a magnesium atom is $ 12. $ so start them ...
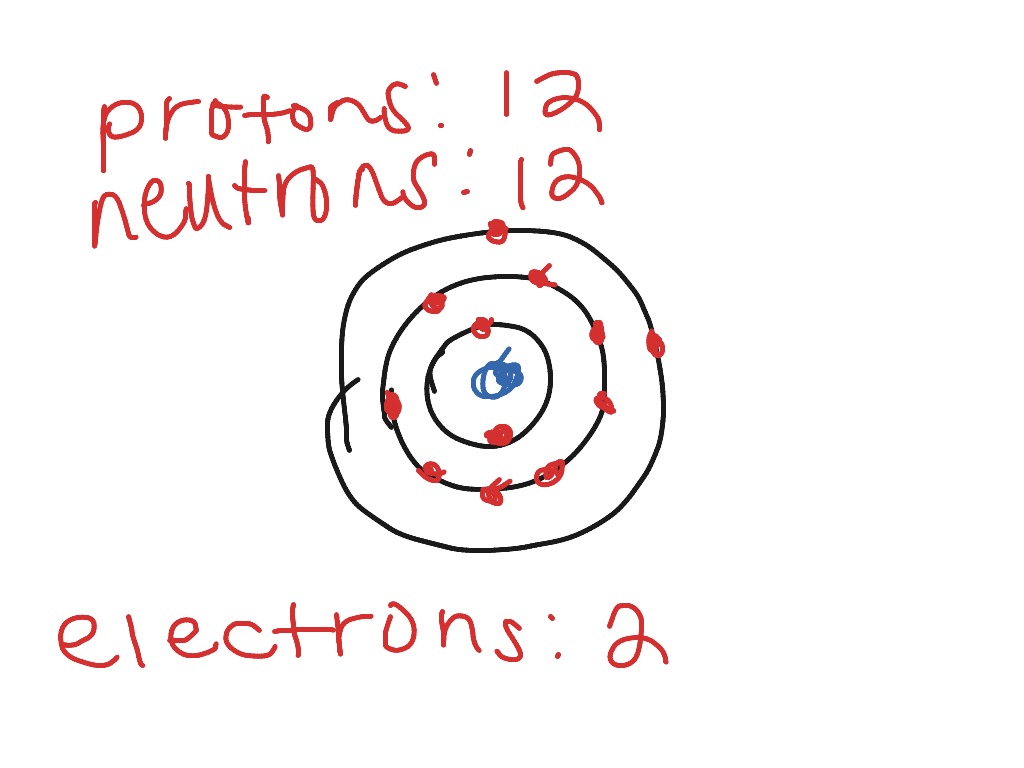
Mg bohr diagram. 14. Scientists use two types of diagrams to show the electron configuration for atoms. Follow your teacher's directions to complete the diagrams. Sulfur Atomic # = 16 Atomic Mass = 32 Protons = 16 Neutrons = 16 Electron = 16 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Mg Atomic ... Bohr Diagram; 10 Mg; 2 pages. 4545060 with drawing_1631504619.pdf. Calhoun Community College. BIO 103. Hazelwood Central High ... The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons. Dec 17, 2015 — Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons. The second holds 8 .1 answer · duch.sd57.bc.ca Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons. The second holds 8. So far, ...
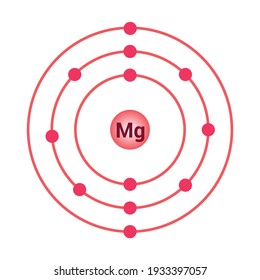
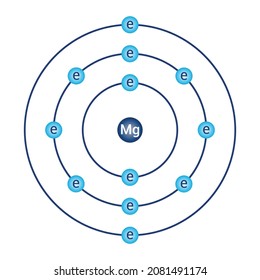
Relative AO Energies for MO Diagrams H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -19.4 eV -15.8 eV -32.4 eV -10.7 eV Photoelectron spectroscopy gives us a pretty good idea of the relative energies for AOs. Mg. Bohr Diagrams. Mg. Magnesium has 3 shells with 12 electrons. Bohr Diagrams. Si. Bohr Diagrams. Si. Silicon has 3 shells with 14 electrons. Bohr Diagrams. Cl. Bohr Diagrams. Cl. Chlorine has 3 shells with 17 electrons. Bohr Diagrams. K. Bohr Diagrams. K. Potassium has 4 shells with 19 electrons. Bohr Diagrams. Ca. Bohr Diagram For Magnesium. A neutral atom has the same number of protons, neutrons, and This diagram shows the electron shell configuration of a magnesium atom. wiringall.com Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons. The second holds 8. Bohr Model for Magnesium by Jackie Moore - October 15, 2013
In a Bohr model, the orbits will have the following maximum electrons: 1st level : 2 e- maximum ... Magnesium has 12 protons based on its atomic number.1 answer · Top answer: An atom is composed of 3 subatomic particles. In the center of an atom, there is the nucleus. • It contains the subatomic particles: protons and neutrons. ... 2005-06-08. Magnesium cation is a Calculi Dissolution Agent and Osmotic Laxative. The mechanism of action of magnesium cation is as a Magnesium Ion Exchange Activity and Osmotic Activity. The physiologic effect of magnesium cation is by means of Inhibition Small Intestine Fluid/Electrolyte Absorption and Increased Large Intestinal Motility and ... Bohr Diagram For Magnesium. Written By Pelvic Diagram Sunday, March 14, 2021. Edit. Bohr Diagram For Magnesium. Vector illustration in flat style with modern long shadow. The phase diagrams are calculated based on the most up-to-date optimized parameters. Magnesium stock illustration. Illustration of formula ... (Billy Carr) Bohr Models of Ions 1. When atoms form an ion, describe the outer shell of that ion. 2. Draw the following Bohr Model Diagrams (NOTE THEY ARE IONS) Be 2+ Cl-F-N3-Ca2+ K+ Na+ O2-Mg2+ Be2+ S2-Li+ Chem WS 6 Page 1
Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.Check me out: http://www.chemistnate.com
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Magnesium, Mg, has 12 electrons distributed as: 1st shell 2 electrons, 2nd shell 8 electrons and third shell 2 electrons.
P=___ N=___ E=___ O S O L O G P _____ _____ M.P. = _____ B.P. = _____ Properties Uses P Bohr Diagram Lewis Structure P=___ N=___ E=___ O S O L O G F _____ _____ M.P
Bohr diagram: Group No. Lewis Dots: Mg: 12: 2 - 8 - 2: 2: 2: N: 7: 2 - 5: 5: 5: Write the Lewis symbols for each atom. See graphic on the left. Determine the numbers of electrons which the atoms will lose and gain by applying the Octet Rule. Mg loses two electrons to have an octet.
Once Bohr had worked out that the energy levels of hydrogen were quan- tized, i.e. In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
A. Cs < Mg < P < F B. Cs < F < Mg < P C. Cs < Mg < F < P D. P < Mg < F < Cs E. F < P < Mg < Cs 9. Rank the following elements in order of increasing atomic size: F, P, Mg, Cs A. F < P < Mg < Cs B. P < Mg < F < Cs C. Cs < F < Mg < P D. Cs < Mg < P < F E. Cs < Mg < F < P 10. Which of the following compounds is likely to have covalent bonds? A. MgO
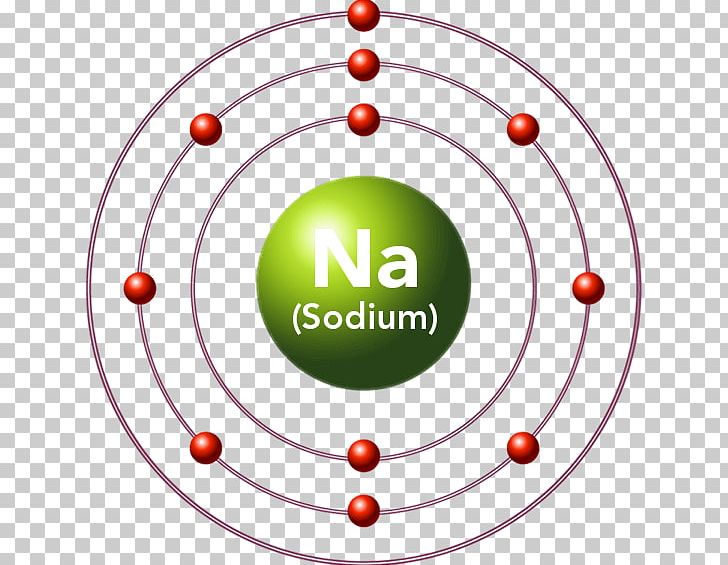
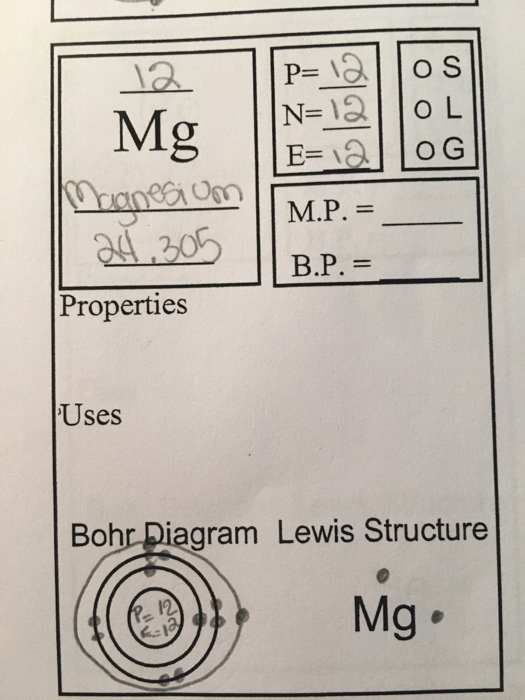
Symbol: Mg Atomic Number: 12 Atomic Mass: 24.305 amu Melting Point: 650.0 °C (923.15 K, 1202.0 °F) Boiling Point: 1107.0 °C (1380.15 K, 2024.6 °F) Number of Protons/Electrons: 12 Number of Neutrons: 12 Classification: Alkaline Earth Crystal Structure: Hexagonal Density @ 293 K: 1.738 g/cm 3 Color: grayish Atomic Structure
Magnesium Chemical Element Bohr Model Diagram Png Clipart Area Atom Bohr Model Chemical Element Chemistry Free
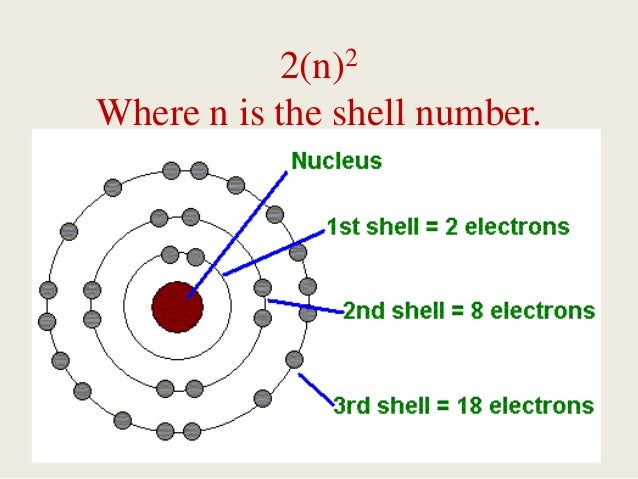
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons ...
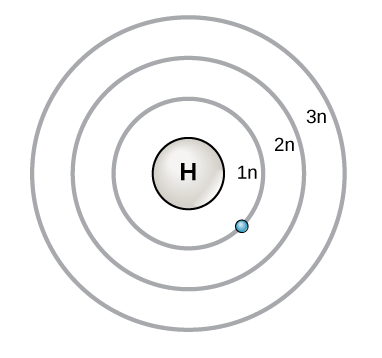
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
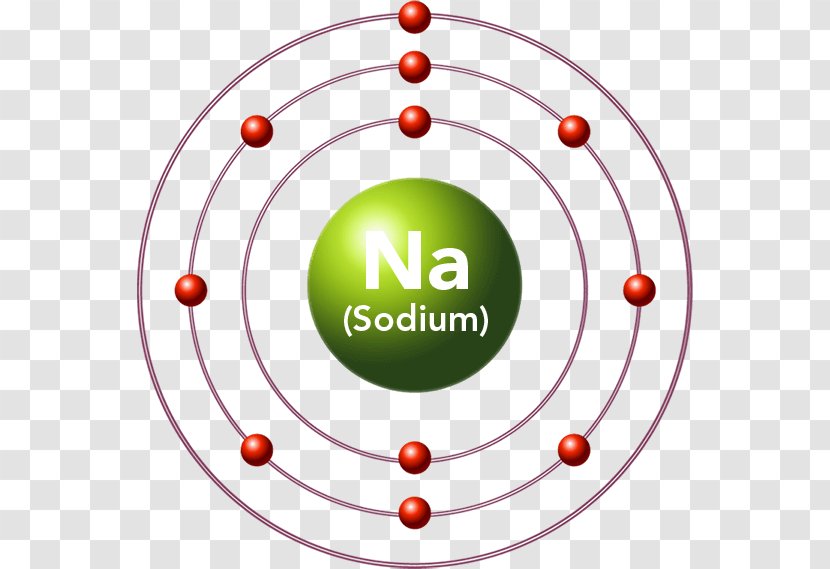
Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.
Magnesium Compounds Are Used In The Production Of Uranium For Nuclear Reactors Draw The Bohr Model For Magnesium Fill Online Printable Fillable Blank Pdffiller
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number. After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells. From the periodic table, find the element, and identify its atomic number and atomic ...
In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ...

Lewis Diagram What Is A Lewis Diagram Simplified Bohr Diagrams Which Only Consider Electrons In Outer Energy Levels Are Called Lewis Diagram A Lewis Ppt Download
Draw Bohr diagrams of the following: (a) O2-(b) Mg2+ (c) Be2-3(d) N - 3. Draw Bohr diagrams of a magnesium atom bonding with fluorine atoms. What type of bonding occurs? Type of bond: _____ 14 . 4. Complete the following table. Note that the name of a NON-METALLIC ion ends in - IDE while the name for a METALLIC ion uses the full name of the ...
Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds two electrons. The second holds 8. So far, 10, of magnesium's 12 ...1 answer · Top answer: Hint :We know that by drawing a nucleus with required number of shells. Since the total number of electrons in a magnesium atom is $ 12. $ so start them ...
Step 7: Draw a Bohr diagram and Lewis Structure to show the arrangement of electrons and the number of valence electrons. ... Green = Li & Na Pink = O & S Blue = Be & Mg Purple = F & Cl Orange = B & Al Red = C & Si Tan = N & P Yellow = He, Ne, & Ar Step 9: Cut the cards apart and arrange according to atomic number in the pattern shown. Once you
Part 1: Bohr Diagrams. 1. Complete the following table by drawing the correct Bohr models for each element. Hydrogen. Lithium. Magnesium. Oxygen. Chlorine.

















Comments
Post a Comment