39 lewis dot diagram for oxygen
Drawing the Lewis Structure for O 2 (Dioxygen or Oxygen Gas). Oxygen (O 2) is a commonly tested Lewis structure due to it's importance on Earth.It also is a good example of a molecule with a double bond. There are 12 valence electrons available for the Lewis structure for O 2.. Video: Drawing the Lewis Structure for O 2 Lewis Electron Dot Structure for the molecule: HCOOH (Formic Acid) In the first step use four electron pairs to form single bonds between neighbouring atoms. Except for hydrogen, complete the octet of each atom by adding two lone pairs on each oxygen atom and one oxygen atom gets double-bonded between C and O.
Answer (1 of 4): The easiest way to them is in steps: Step 1: Count number of total Valance electrons (12 electrons in this case) Step 2: No. of Required electrons (always 8 hence, 16 electrons) Step 3: No. of Bonding Electrons (Required electrons - valence electrons: 4 electrons in this case)...

Lewis dot diagram for oxygen
O2 Lewis structure oxygen electron dot structure is that type of diagram where we show the total 12 valence electrons of O2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers.
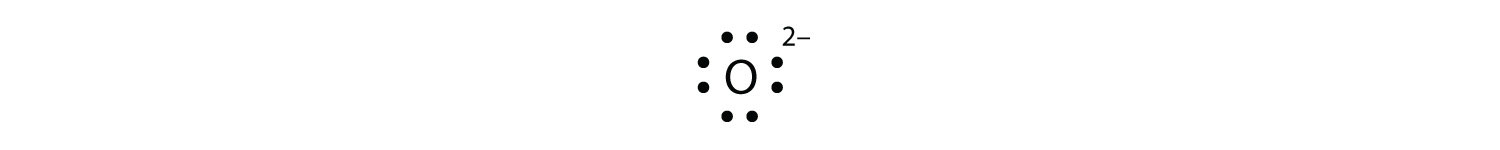
Lewis dot diagram for oxygen. A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom. Example A. Determine the total number of valence electrons for C. So, according to the lewis dot structure of OF2, oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. ∴ OF2 formula becomes AX 2 N 2. According to the VSEPR chart, the molecule which has the AX 2 N 2 formula their molecular shape is bent and electron geometry is tetrahedral.
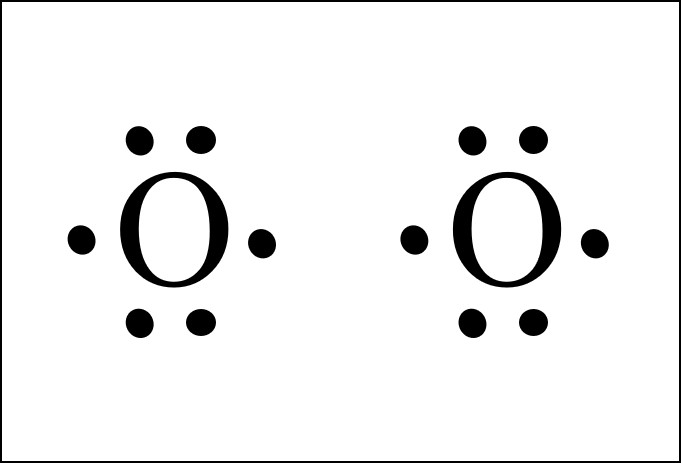
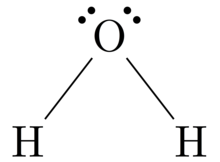
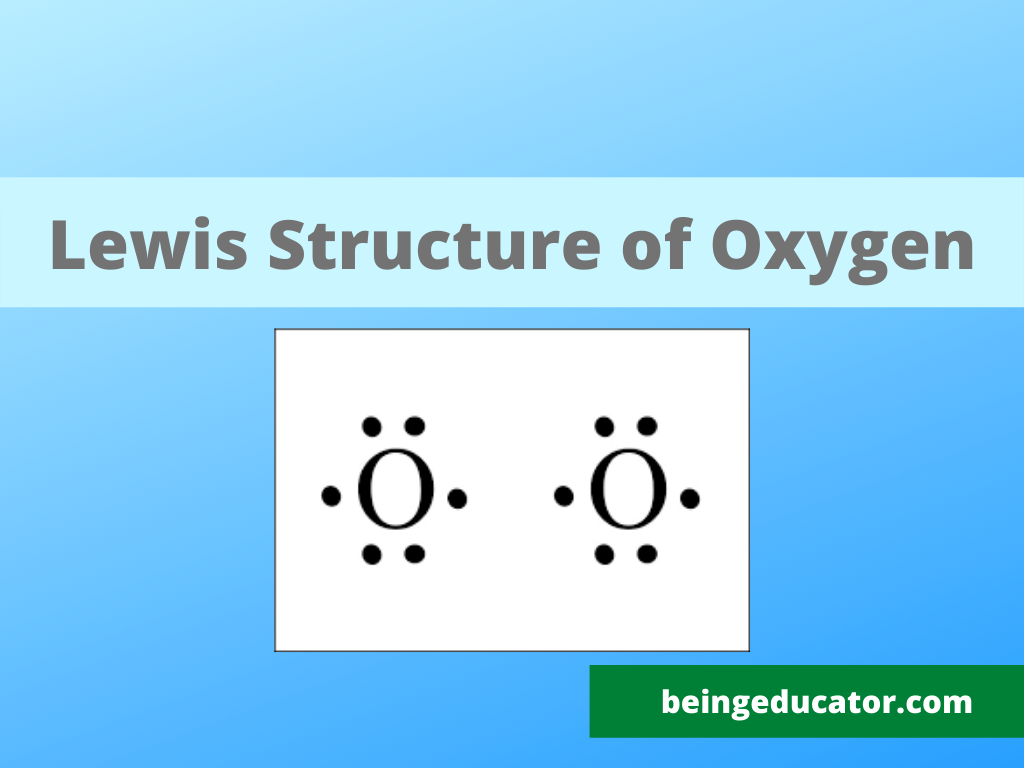
The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. The Lewis Dot Structure for O2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for O2, commonly referred to as oxygen gas. Due to oxygen's high electronegativity (affinity for electrons), the pure element is nearly exclusively found in either this state or ozone (O3 - a distinct lewis structure for another post). For example, consider the Lewis dot structure for carbon dioxide. This is a linear molecule, containing two polar carbon-oxygen double bonds. However, since the polar bonds are pointing exactly 180° away from each other, the bond polarities cancel out, and the molecule is nonpolar. The Lewis structure for oxygen (O2) shows the bond between two oxygen atoms. Each has a total of 6 valence atoms making a sum total of 12. The two oxygen atom can both achieve a stable structure by sharing two pairs of electrons. The double bond they share is denoted by the double lines joining the two atoms.
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. Lewis Structure (electron dot diagram) for the oxygen molecule, O 2, OR . There are 2 bonding pairs of electrons shared between the 2 oxygen atoms, and each oxygen atom also has 2 lone pairs (non-bonding) pairs of electrons. (d) Xenon can react with oxygen and fluorine to form compounds such as XeO 3 and XeF 4. In the boxes provided, draw the complete Lewis electron-dot diagram for each of the molecules represented below. XeO 3 XeF 4 One point is earned for each correct Lewis electron-dot diagram. Omission of lone pairs of electrons on the O or F atoms Lewis Dot Structure: The lewis dot structure of the oxygen atom can be represented by the pictorial diagram by showing the number of valence electrons with dots around the symbol of the oxygen.
For diatomic oxygen, the Lewis dot structure predicts a double bond. While the Lewis diagram correctly predict that there is a double bond between O atoms, it incorrectly predicts that all the valence electrons are paired ( i.e. , it predicts that each valence electron is in an orbital with another electron of opposite spin).
Photo: Benjah-bmm27 via Wikimedia Commons, Public Domain. O2 is an allotrope of oxygen and is made out of two oxygen atoms bound together. Although the chemical formula for this allotrope is O2, it is frequently just referred to as oxygen.O2 or dioxygen's particular formulation is one of the most common elemental compounds on the planet, constituting around 20.8% of the Earth's atmosphere.
Follow some steps for drawing the lewis dot structure of BrO3-1. Count total valence electron in BrO3-Lewis diagram is a simple representation of valence electron within a molecule. So, for determining the valence electron in BrO3-, look at the periodic group of bromine and oxygen atoms. ... Each oxygen atom in the 5th step structure has 3 lone ...
Live. •. See the Big List of Lewis Structures. Transcript: OK, we're going to do the Lewis dot structure for O2. Let's start. Looking on the periodic table, we can find Oxygen in group 6 or 16, and that means it has 6 valence electrons. But we have two of them so we'll multiply that by 2. That gives us a total of 12 valence electrons.
A step-by-step explanation of how to draw the Lewis dot structure for O (Oxygen). I show you where Oxygen is on the periodic table and how to determine how ...
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t...

Carbon Has Four Valence Electrons And Oxygen Has Six Valence Electrons If Carbon And Oxygen Bond Brainly Com
Lewis dots are diagrams showing the bonding between atoms of a molecule and the remaining lone pairs of electrons in the molecule. The Lewis dot structure for oxygen is O with two dots on the left ...
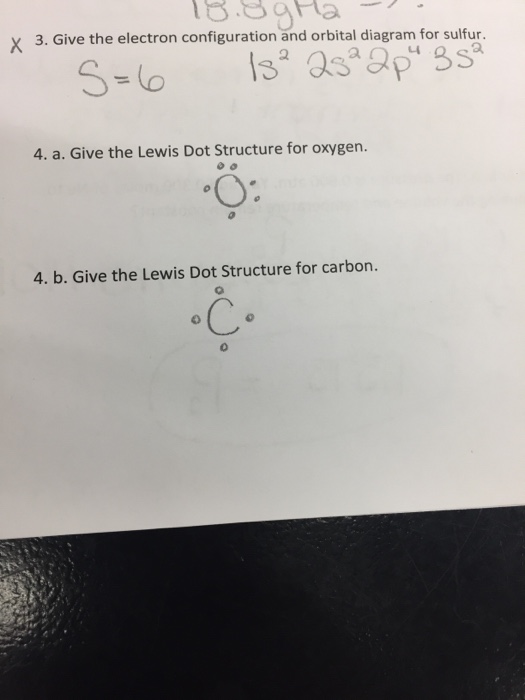
The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom.
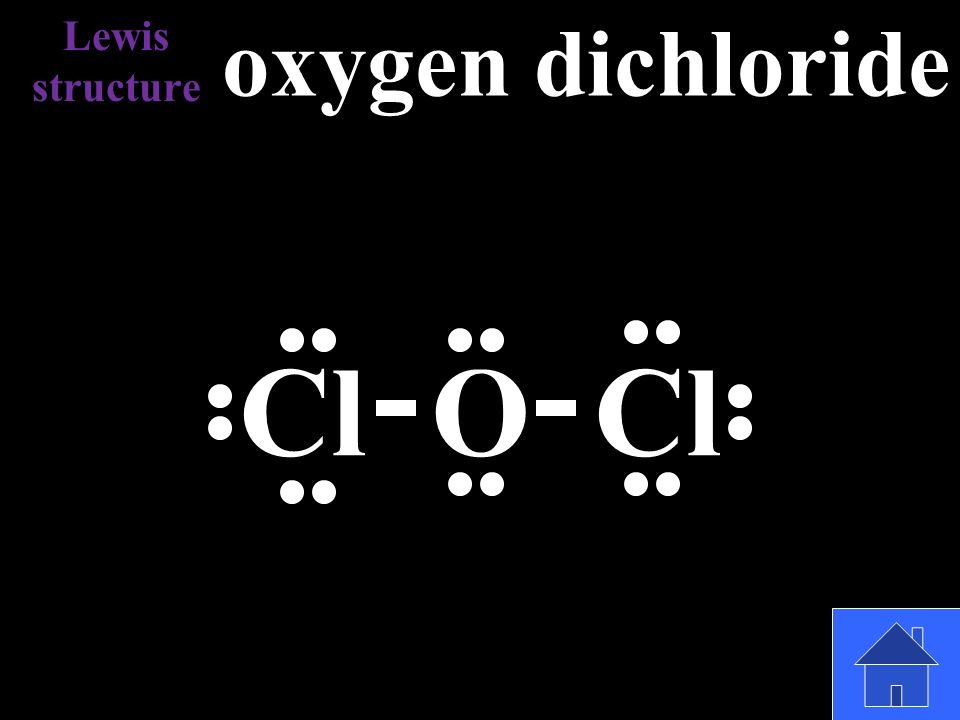
2 Answers. O goes in the middle. First, that's the order they are written in. Second, oxygen forms 2 bonds, so it can form a bond with H and with I. Starting with that, finishing the Lewis structure is very easy. Just account for 1 + 6 + 7 = 14 valence electrons in the drawing. Kleiner.
O2 Lewis Structure Setup. It's easiest to think in terms of dots to make the O 2 Lewis structure. Oxygen needs to bond twice, shown as the lone dots on the left and right sides of the oxygen atoms in the below diagram. There are also two pairs of dots, representing four more electrons, that won't bond.
the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. What is the Lewis dot structure for Ce? what is the dot diagram for Ce
The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers.
Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen.
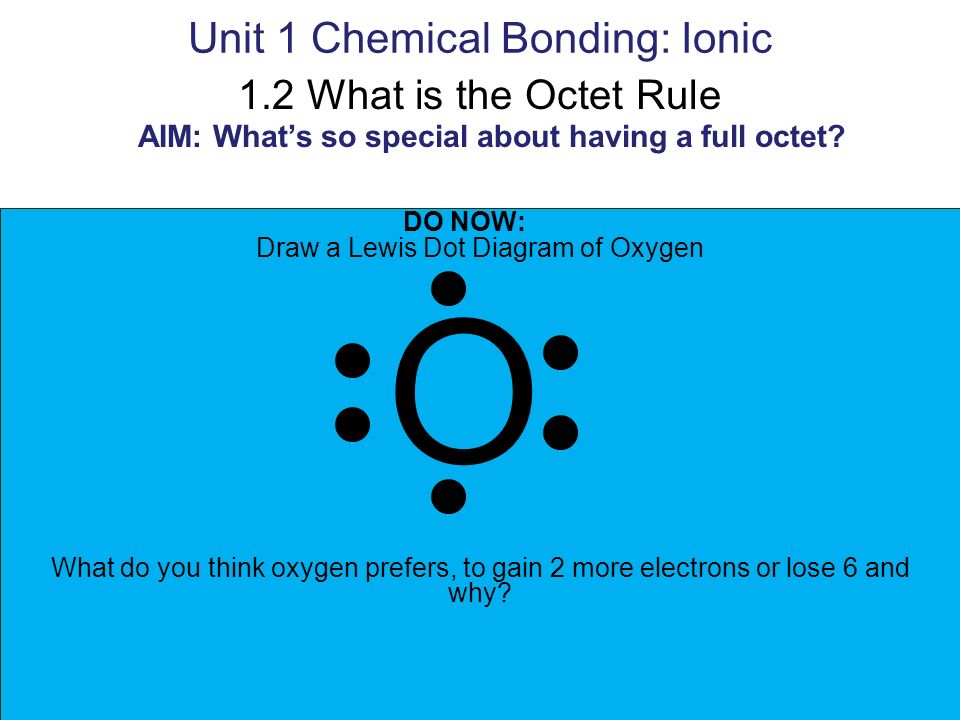
Unit 1 Chemical Bonding Ionic 1 2 What Is The Octet Rule Aim What S So Special About Having A Full Octet Do Now Draw A Lewis Dot Diagram Of Oxygen Ppt Download
O2 Lewis structure oxygen electron dot structure is that type of diagram where we show the total 12 valence electrons of O2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots.

Question 1 I Write The Electron Dot Structures For Sodium Oxygen And Magnesium Ii Show The Formation Of Na2o And Mgo By The Transfer Of Electrons Iii What Are The Ions Present In
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts
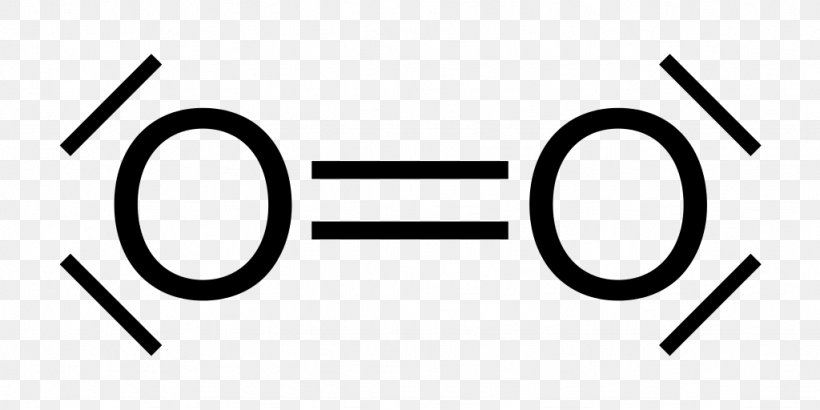
Lewis Structure Dioxygen Molecule Singlet Oxygen Png 1024x512px Lewis Structure Area Atom Bicarbonate Black And White





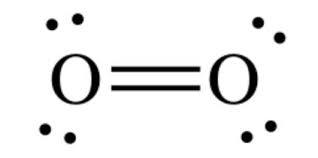










Comments
Post a Comment