38 bohr diagram for chlorine
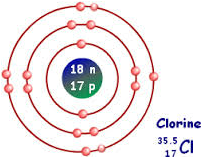
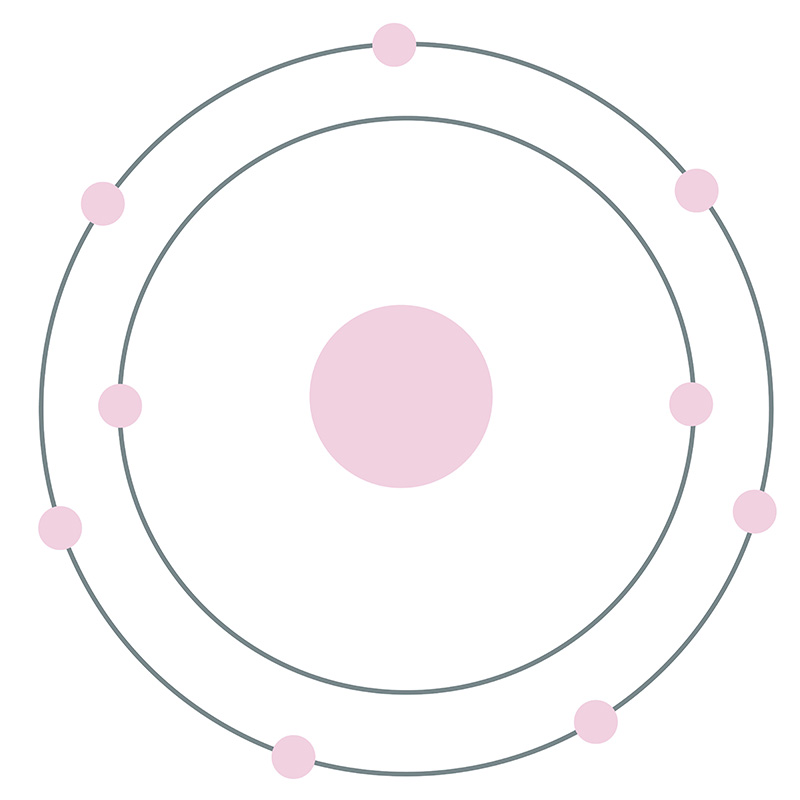
Jul 6, 2018 — This is a Bohr model of a chlorine-35 atom. Some Bohr models pair six of the seven electrons in the third (valence) shell.2 answers · No I don't believe so. Explanation: The two electrons in the first shell should be together ... Sep 01, 2021 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.

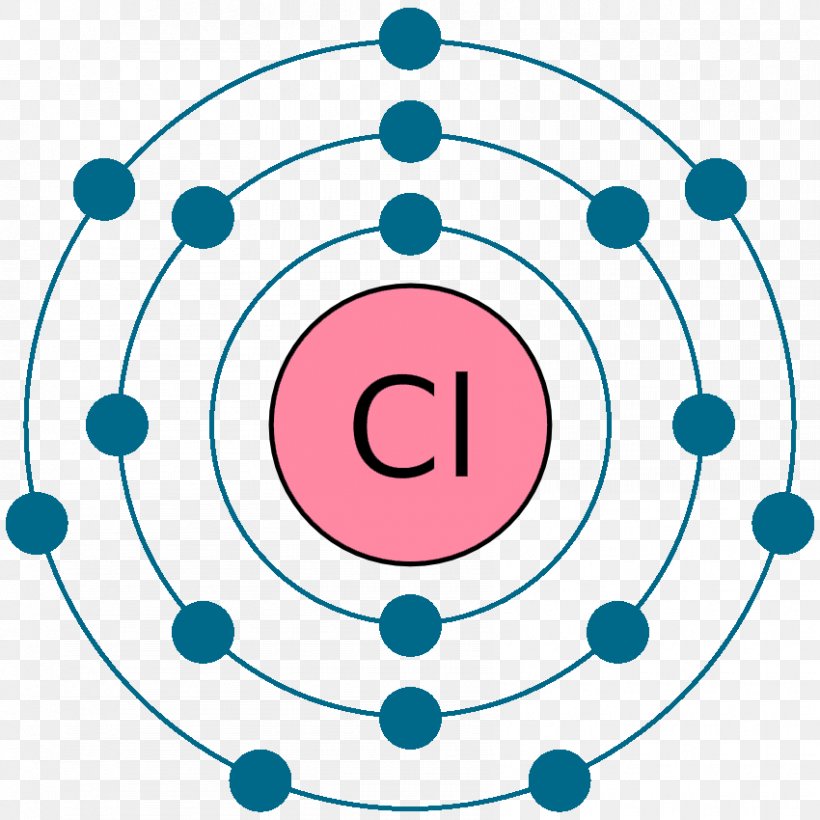
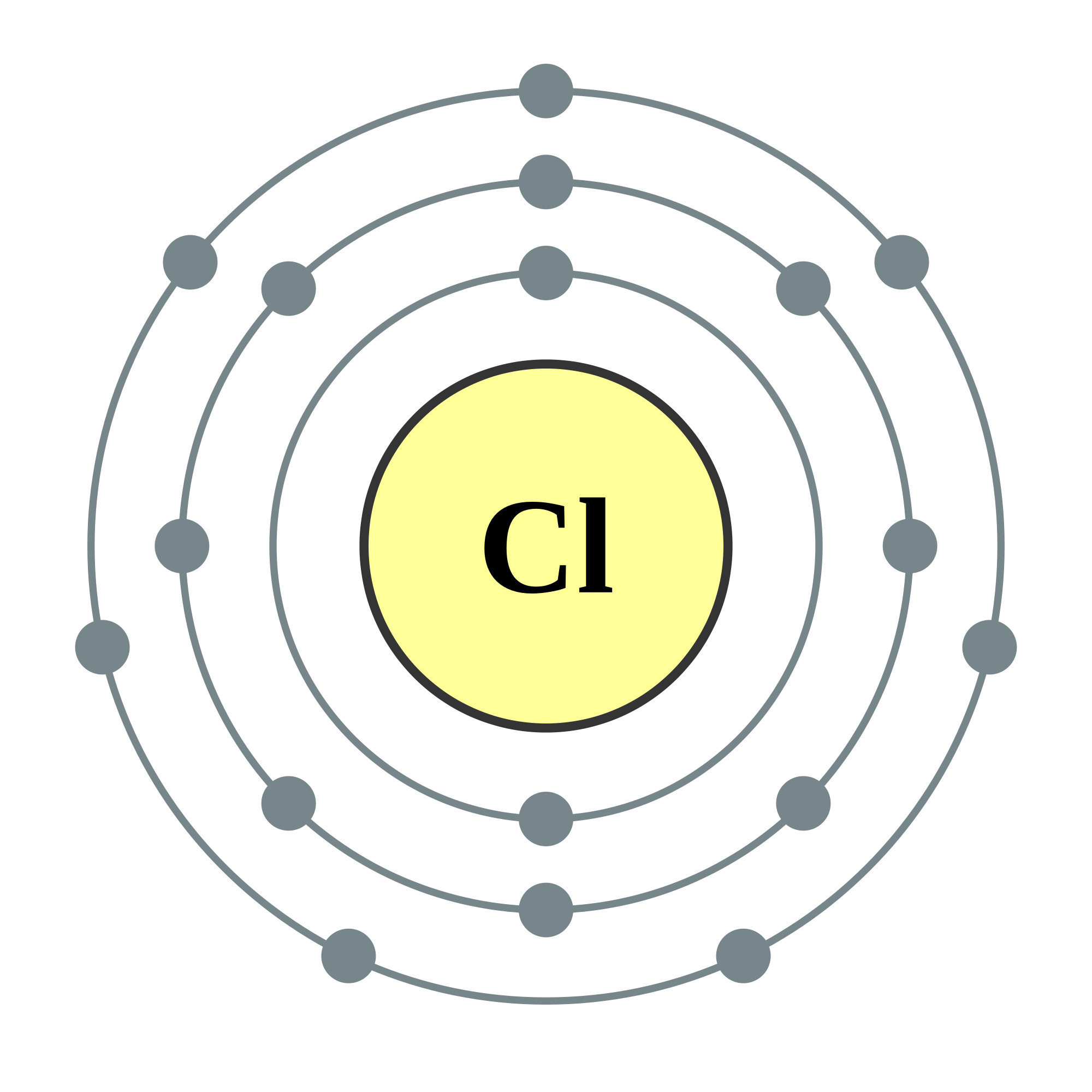
Bohr diagram for chlorine
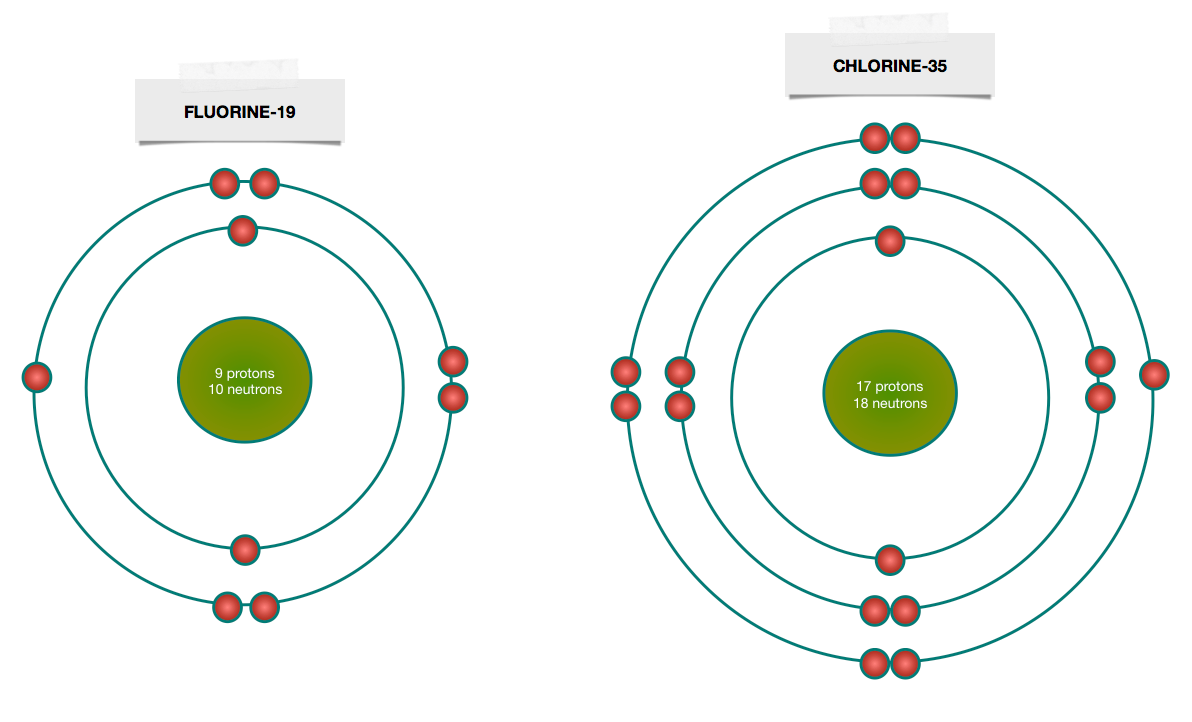
If you look at the diagrams of the sodium and chlorine atoms you can see that sodium normally has eleven electrons in shells around the nucleus. Bohr Diagram. Be. Bohr Diagram. Cl. Bohr Diagram Physical Science Name/Per/Due date_____ Valence Electron Practice. Directions: Give the total number of electrons and the number of valence electrons for each element listed below. Hydrogen 2. Lithium. Beryllium 4. Carbon. Fluorine 6. Neon. Magnesium 8. Chlorine. Arsenic 10. Krypton. Barium 12. Draw a Bohr Model of Chlorine (Cl) Atomic Number: 17 (# of protons & therefore, same # of electrons) Atomic Mass: (Atomic mass – Atomic number = # of ...
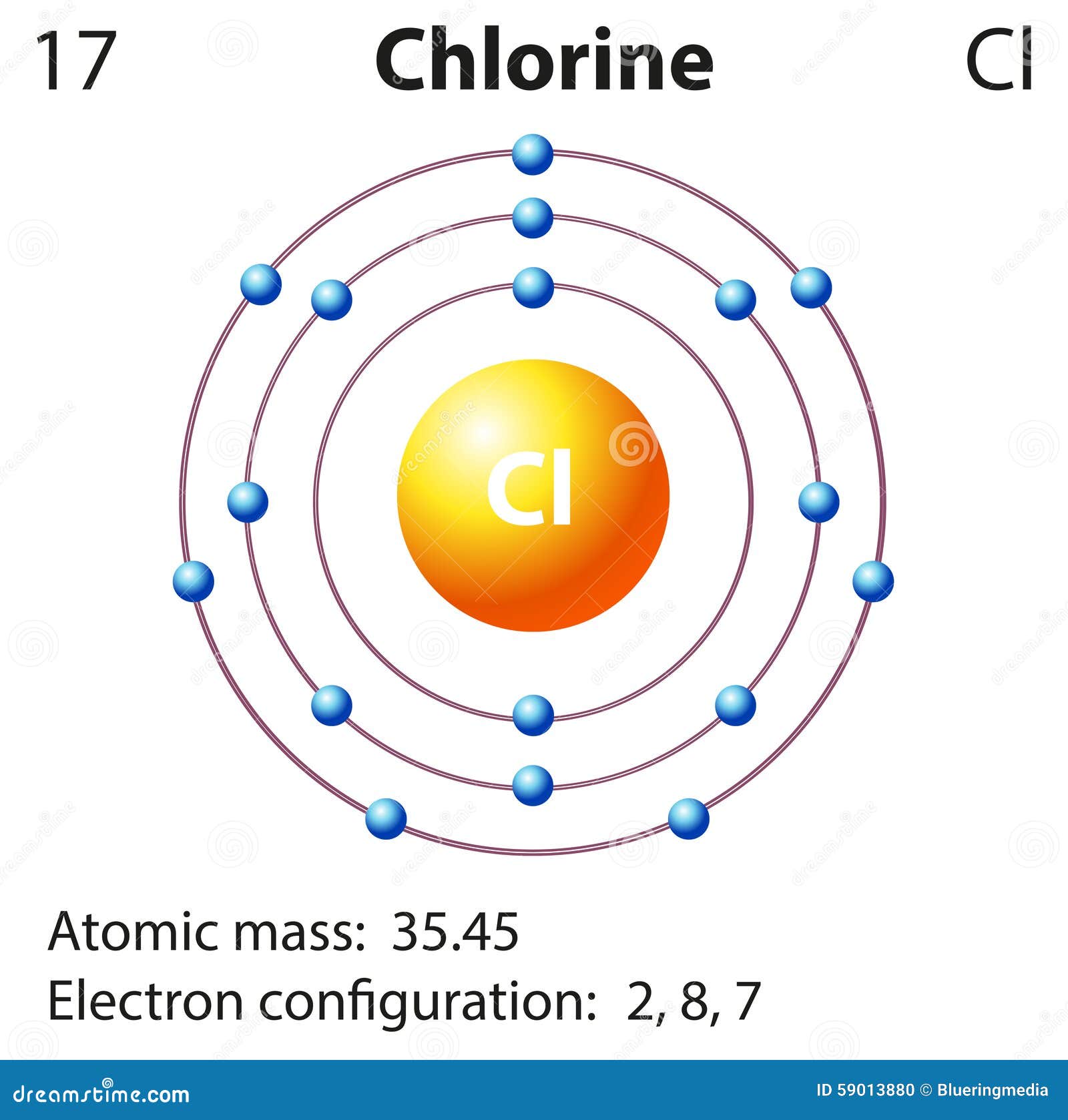
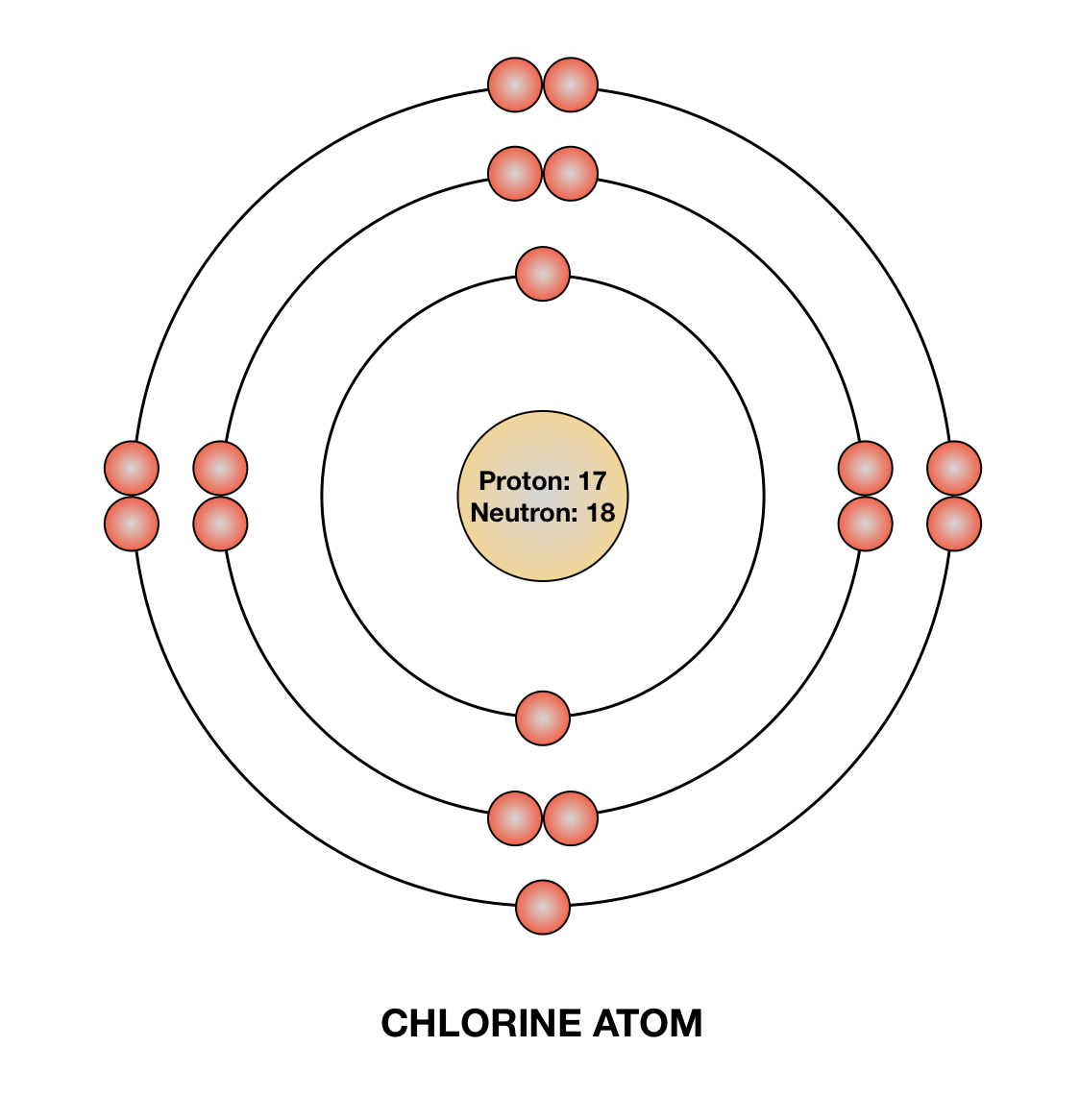
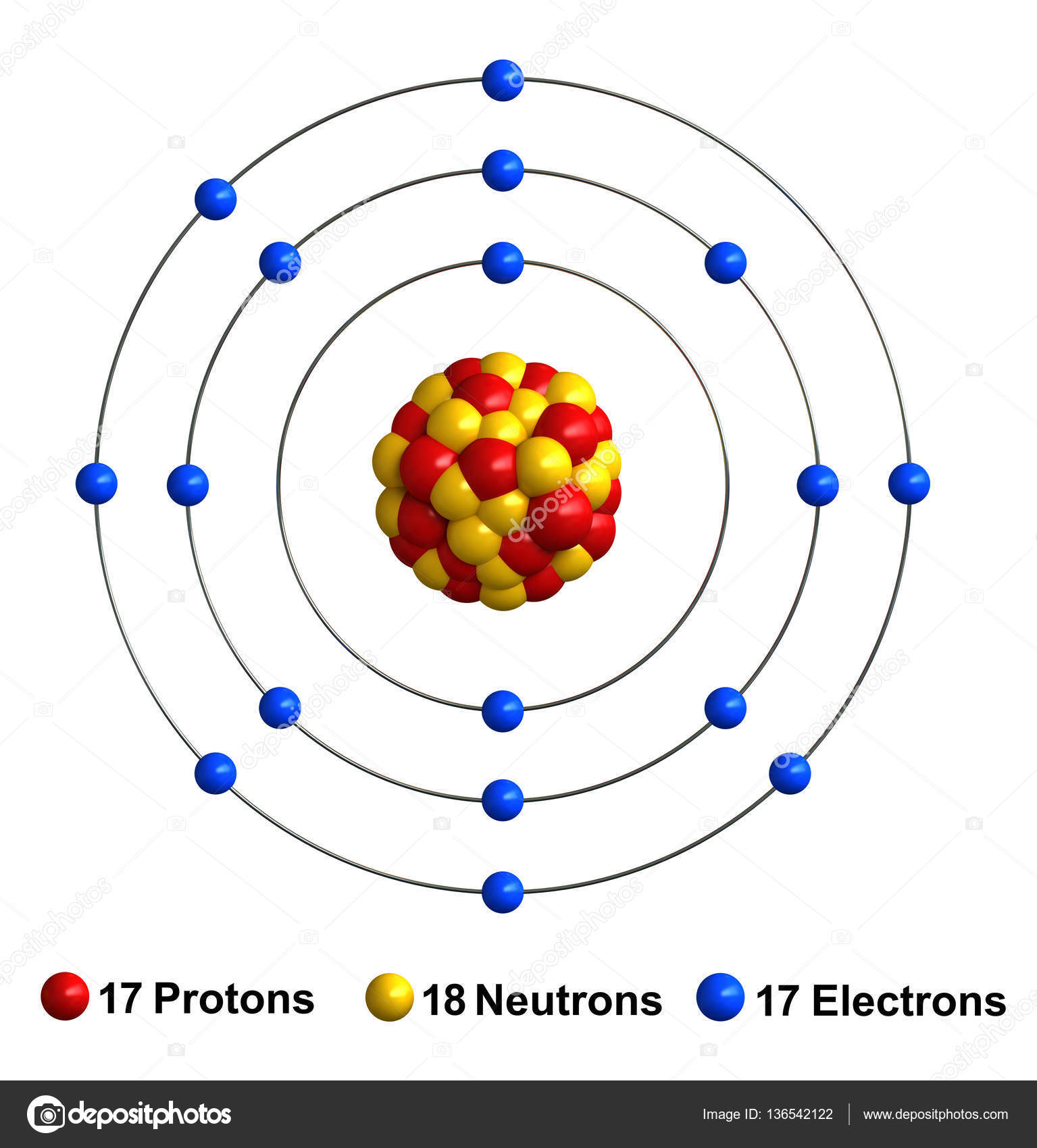
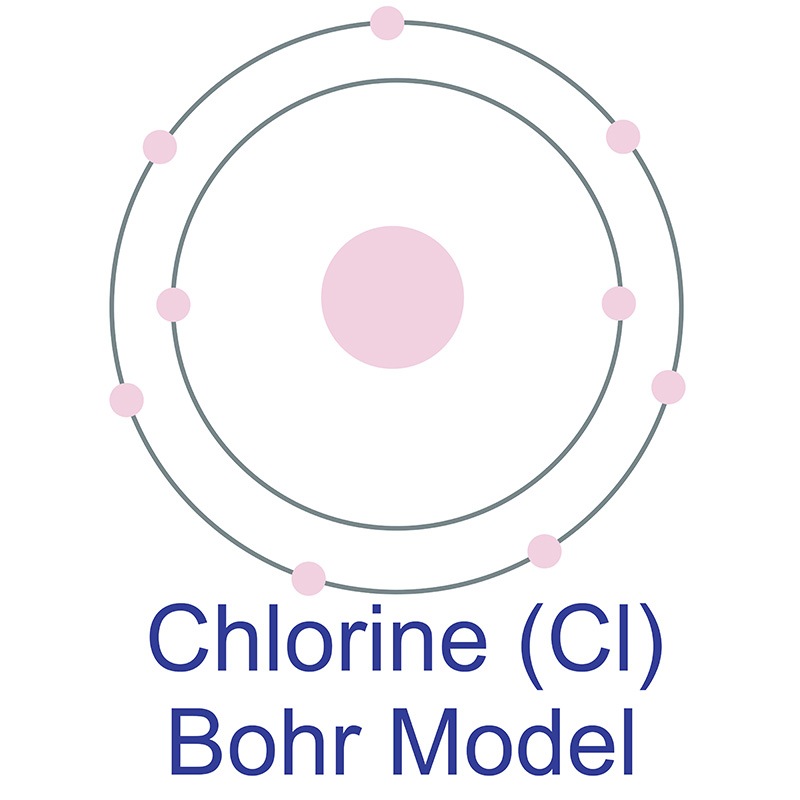
Bohr diagram for chlorine. Draw a Bohr Model of Chlorine (Cl). Atomic Number: 17. (# of protons & therefore, same # of electrons). Atomic Mass: 35.453. Name: Chlorine Symbol: Cl Atomic Number: 17 ... Number of Protons/Electrons: 17. Number of Neutrons: 18 ... [Bohr Model of Chlorine] Sep 22, 2021 · In the Bohr Model of the atom, electrons occupy fixed orbits around the nucleus called energy levels. However in the Quantum Mechanical Model of the atom, electrons occupy orbitals. Orbitals are grouped by size and shape into shells and subshells (or, levels, and sublevels). Bohr Model Drawing Draw a Bohr model of a chlorine atom in the space below. Be sure to place the electrons in the correct orbitals and to fill out the key for the subatomic particles. Protons: Neutrons: Electrons: Chlorine 35.42 Atomic number equals the number of or Atomic mass equals the number of Identify the each of the parts of the box. Oxygen
Now it resembles neon with 8 electrons in the outer shell. When it was neutral, chlorine had 17 electrons and 17 protons. When it gains 1 electron, it has 18 ...3 pages Draw and explain a Bohr diagram and a Lewis dot diagram for chlorine. View Answer In the hydrogen atom, what is the electric potential energy of the electron when it is found in the n = 4 state? The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17 electrons (green) bind to the nucleus, successively occupying available electron shells ( ... Aug 15, 2020 — Bohr diagrams show electrons orbiting the nucleus of an atom somewhat ... In contrast, chlorine and sodium have seven and one electrons in ...
Free Online CBSE Class Notes, NCERT Solutions, Self Study material for Class 6, Class 7, Class 8, Class 9, Class 10, Class 11 and Class 12 Draw a Bohr Model of Chlorine (Cl) Atomic Number: 17 (# of protons & therefore, same # of electrons) Atomic Mass: (Atomic mass – Atomic number = # of ... Bohr Diagram. Be. Bohr Diagram. Cl. Bohr Diagram Physical Science Name/Per/Due date_____ Valence Electron Practice. Directions: Give the total number of electrons and the number of valence electrons for each element listed below. Hydrogen 2. Lithium. Beryllium 4. Carbon. Fluorine 6. Neon. Magnesium 8. Chlorine. Arsenic 10. Krypton. Barium 12. If you look at the diagrams of the sodium and chlorine atoms you can see that sodium normally has eleven electrons in shells around the nucleus.














Comments
Post a Comment