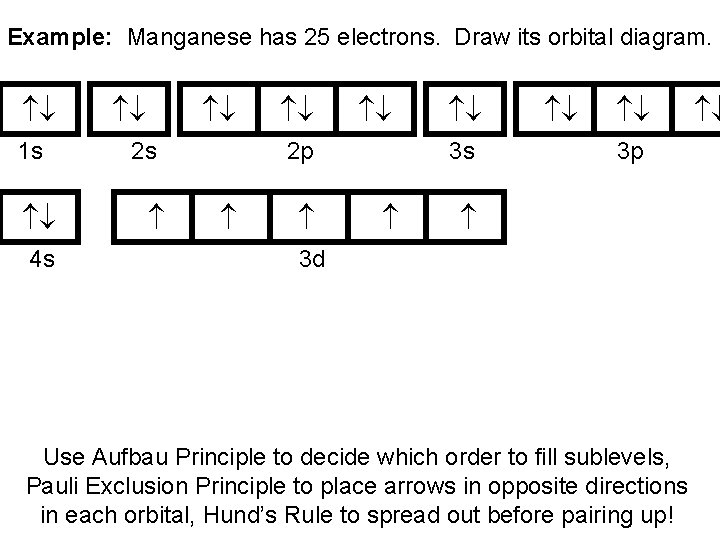
43 orbital diagram for mn
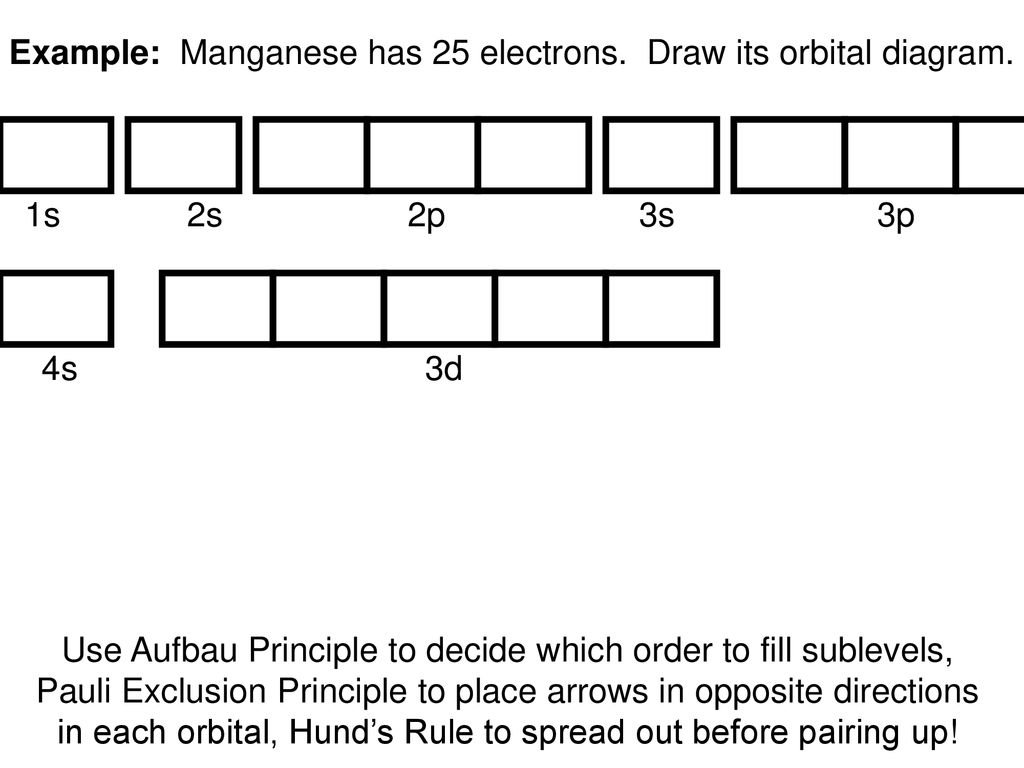
25 Mn 55 = [Ar] 4s 2 3d 5. Diagram Orbital Konfigurasi Elektron Unsur Mangan 25 Mn. Diagram orbital untuk konfigurasi electron pada subkulit 4s dan 3d unsur mangan dapat dilihat pada gambar berikut. Contoh Soal Menentukan Bilangan Kuantum Elektron Unsur Mangan Mn. Start studying Electron Configurations and Orbital Diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Fig. 1: Structure an doping of the prototype system. Fig. 2: The experimental phase diagram of the AMn 7 O 12 series, plotted as a function of formal B-site valence, constructed from 21 samples ...
Orbital diagram for mn
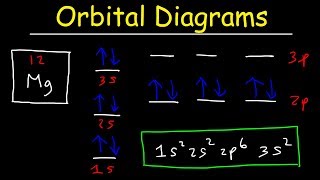
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... 11 Apr 2021 — The ground state electron configuration of ground state gaseous neutral manganese is [Ar]. 3d5. 4s2 and the term symbol is 6S5/2. 29 Jul 2016 — The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . The diagram below represents the electron configuration ...1 answer · Refer to the explanation. Explanation: The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents ...
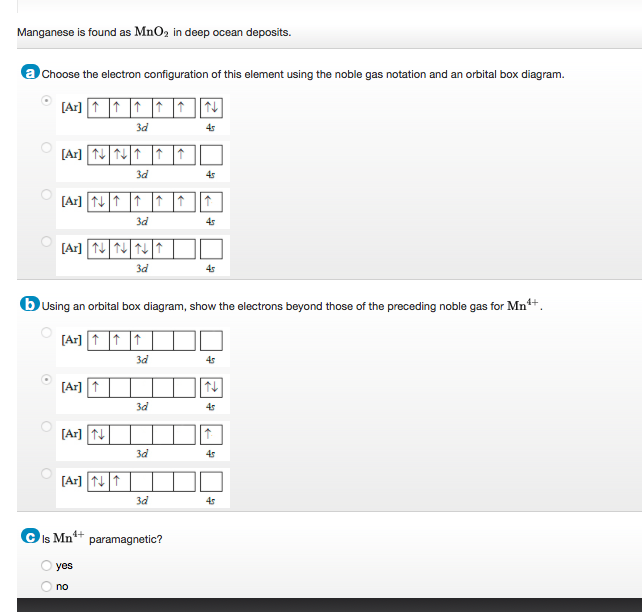
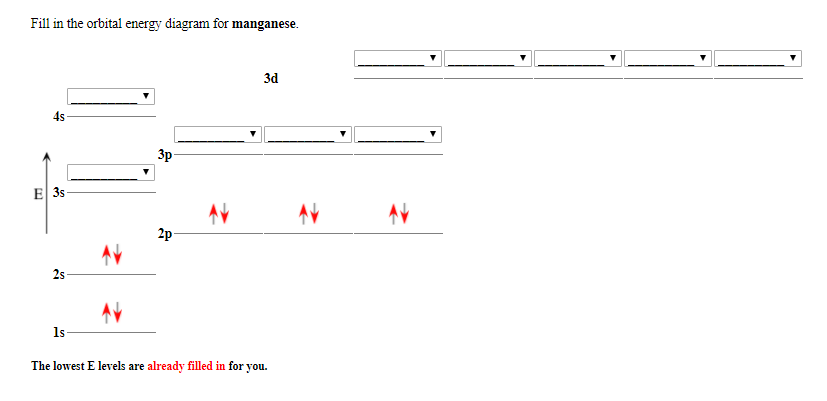
Orbital diagram for mn. Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36 ... The electron configuration for manganese is 1s2 2s2 2p6 3s2 3p6 4s2 3d5. It can be shortened to [Ar] 4s2 3d5, where the [Ar] represents argon, the last element in the third row of the periodic table, whose electrons fill every shell prior to the 4s-orbital. The first number in each grouping represents the energy level. • Mn(acac) 3 is green by transmitted light, owing to a single broad d-d transition at about 500 nm. • KMnO 4 is deep purple, owing to a charge transfer transition with high molar absorptivity.!The ligand (acac) in the product is the anion of acetylacetone, 2,4-pentanedione, CH 3COCH 2COCH 3, Hacac.!Mn(acac) 3 has octahedrally coordinated Mn ... the 4s orbital is lower in energy than the 3d orbital for main group elements. however for transition metals, the 3d orbital is lower than the 4s, thus 4s electrons will be lost first. hence Mn2 has a E.C of [Ar] 3d5 4s0
When placing electrons in orbital diagrams, electrons are represented by arrows. An arrow pointing up corresponds a spin of +1/2 and an arrow pointing corresponds to a spin of -1/2. Electrons in different singly occupied orbitals of the same sub-shell have the same spins (or parallel spins, which are arrows pointing in the same direction). The sub-shell relates to the s, p, d, and f blocks ... a) [MnCI 6] 4-By drawing the molecular orbital energy diagram of the Mn-Cl π-interaction in the complex ion, show how the cleavage energy of the crystal field will be affected on the figure. (Mn: 25) b)[Pb(CN) 6] 2- Draw the molecular orbital energy diagram according to the σ - interaction of the complex ion. manganese mn chemicalaid manganese mn has an atomic mass of 25 find out about its chemical and physical properties states energy electrons oxidation and more. Hf Molecular Orbital Diagram – Orbital Diagram For Fluorine Awesome 0d Mos2 2d G. arrangements of electrons in the orbitals of an atom is the orbital diagram the electron configuration ... Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quantum number used to distinguish between the electrons in an orbital. He (Z = 2): 1s 2. The third electron goes into the next orbital in the energy diagram, the 2s orbital. Li (Z = 3): 1s 2 2s 1 a) Mn(OH 2) 6 2+ b) Fe(CN) 6 4- c) Co(NH 3) 6 3+ Other Geometries. Crystal field theory has also been applied to other geometries of coordination compounds. Once again, in terms of the d orbital splitting diagram, the results are similar to what we see from molecular orbital theory. For tetrahedral geometry, which is the most common geometry ... Angular momentum l (orbital shape) Magnetic m l (orbital orientation) These 3 quantum numbers are the spatial quantum numbers. ⇒ together, they describe the 3D appearance of the orbital in space ⇒ the spatial probability distribution of an e-described by that orbital The 4th quantum number is necessary to fully describe an e-in an orbital.
Answer (1 of 10): [Mn(CN)6]^3- is composed of six CN- ions and Mn^3+ ion. The electronic configuration of Mn^3+ ion is [Ar] 3d4. As CN- is a strong field ligand, two electrons are paired up. Two vacant 3d orbitals ( dx2-y2 and d z2), one 4s orbital and three 4p orbitals are hybridized and leads t...

Which Orbital Diagram Represents The Electron Configuration Of High Spin Octahedral Complex Cr Nh3 6 2 Select The Cor Homeworklib
its a picture god you stupid people..... Wiki User. ∙ 2012-02-29 15:53:09. This answer is:
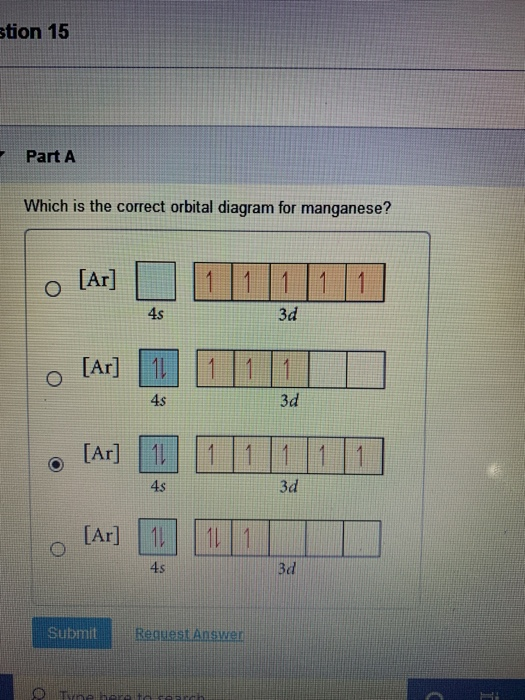
Identify the orbital (4 leaf clover) A) px orbital B) dxy orbital C) py orbital ... A) F B) Li C) Cl D) Na. C) 1s^22s^22p^63s^23p^64s^23d^5. Give the complete electronic configuration for Mn. A) 1s^22s^22p^63s^23p^64s^24p^5 B) 1s^22s^22p^63s^23p^64s^13d^6 C) 1s^22s^22p^63s^23p^64s^23d^5 ... Choose the orbital diagram that represents the ground ...
What is the orbital notation for Mn? The notation would be 1s22s22p63s23p64s23d5, or in noble gas notation, [Ar] 4s23d5.
28 Jul 2021 — Electron Configuration for Mn · With the Aufbau principle, the first orbital has 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on electrons. · Subsequently, ...
Most abundant ores are pyrolusite (MnO 2), psilomelane [(BaH 2 O) 2 Mn 5 O 10] and rhodochrosite (MnCO 3). Annual world production is around 6,220,000 tons. Primary mining areas are South Africa, Russia, Gabon, Australia, Brazil. Uses of Manganese: Used in steel, batteries, axles, rail switches, safes, plows and ceramics. Additional Notes ...
In the second diagram only sigma bonding is considered and it shows the combination of the metal 3d, 4s and 4p orbitals with OCCUPIED ligand group orbitals (using 1 orbital from each ligand). The result is that that the metal electrons would be fed into t2g and eg* molecular orbitals which is similar to the CFT model except that the eg orbital is now eg*.
So if you look at the periodic table, Manganese is going to have an electron configuration of one S 2. She was too two P six, three S two, three, P six 45. And ...1 answer · Top answer: You can determine the ground-state electron configuration of Manganese (Mn) by referring to the periodic table and locating the position of Mn in the ...
Solution for Mn^2+orbital filling diagram. Q: A friend tells you: " The constant K sp of a salt is called the sol ubility product constant and is ... A: Solubility product is used only for those salts that are sparingly soluble and there exists equilibr...
#"Mn: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^5 4s^2# Now, it's very important to remember that the #2# electrons that are lost when the manganese(II) cation is formed are coming from the #4s# orbital, which is higher in energy than the #3d# orbitals when filled. This means that the electron configuration of the manganese(II) cation will be
A ground-state atom is an atom in which the total energy of the electrons can not be lowered by transferring one or more electrons to different orbitals. That is, in a ground-state atom, all electrons are in the lowest possible energy levels. eg: Consider a carbon atom whose electron configuration is the following.
Manganese (Mn) has an atomic mass of 25. Find out about its chemical ... Electron Configuration, [Ar] 4s2 3d5. 1s2 2s2 2p6 3s2 3p6 4s2 3d5. Orbital Diagram.Atomic Number: 25Atomic Weight: 54.938045 Isotopes
Our results and analysis allowed a near-complete binding-energy-scaled MnO4-(aq.) molecular orbital and a valence energy level diagram to be produced for the MnO4-(aq.)/MnO4˙(aq.) system.
The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral ... [Mn(H2O)6]2+ is generated when seventeen electrons (five from Mn2+ and twelve electrons from six aquo
29 Jul 2016 — The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . The diagram below represents the electron configuration ...1 answer · Refer to the explanation. Explanation: The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents ...

Solved 6 4 Pts Which Ofthe Following Orbital Diagrams Listed Below Best Describes The Ground State Electron Configuration Of Mn Which One Is The Orbital Diagram For Mnt Ar 3d A D 3d
11 Apr 2021 — The ground state electron configuration of ground state gaseous neutral manganese is [Ar]. 3d5. 4s2 and the term symbol is 6S5/2.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...

Figure 4 From Molecular Orbital Scf Xc Sw Theory Of Fe 2 Mn 3 Fe 3 Mn 2 And Fe 3 Mn 3 Charge Transfer And Magnetic Exchange In Oxides And Silicates Semantic Scholar

Mn Manganese Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
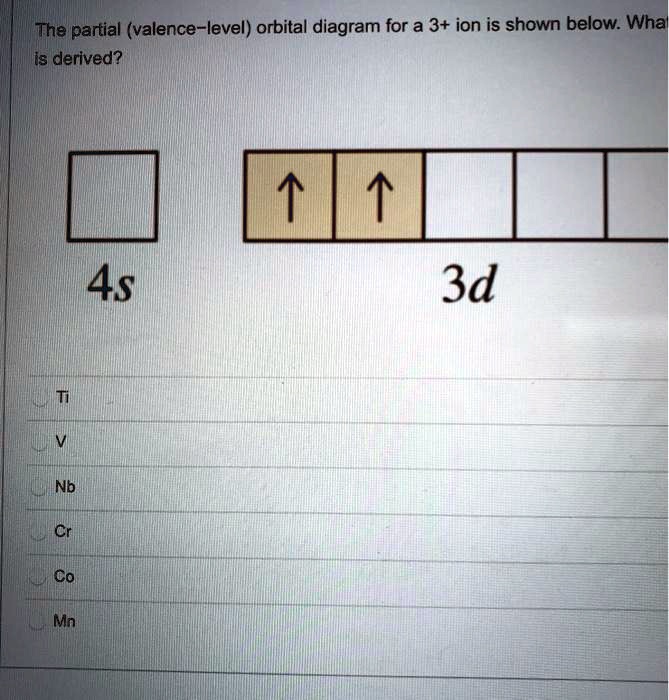
Solved The Partial Valence Level Orbital Diagram For A 3 Ion Is Shown Below Wha Is Derived 4s 3d Nb Co Mn

Welcome To Chem Zipper Com Why Mn H2o 6 2 Is Colourless Although In Which Mn 2 Ion Had Five Unpaired Electrons
Consider The Molecule Mnx6 2 Where X Is A Neutral Ligand Assume That X Is A Strong Field Ligand Socratic

Solved Use An Orbital Diagram To Describe The Electron Configuration Of The Valence Shell Of Each Of The Following Atoms Let Stand For The Highest Occupied Principal Quantum Level And M For The

















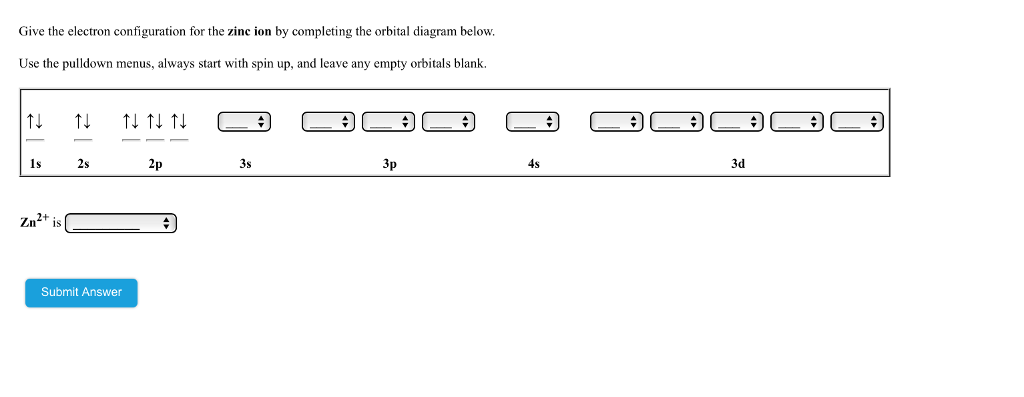
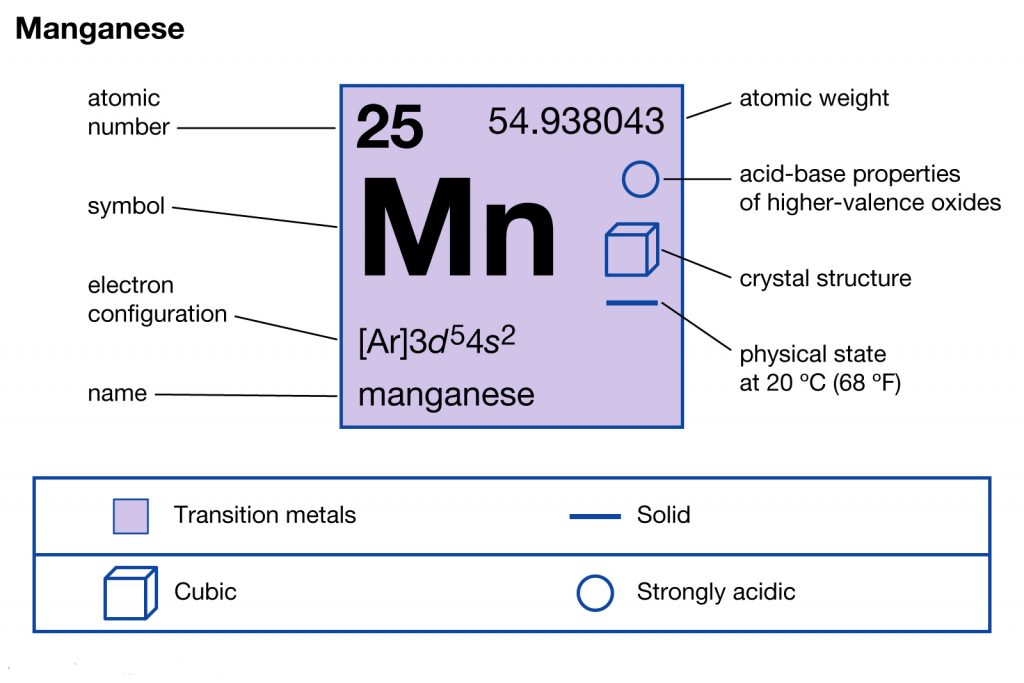
Comments
Post a Comment