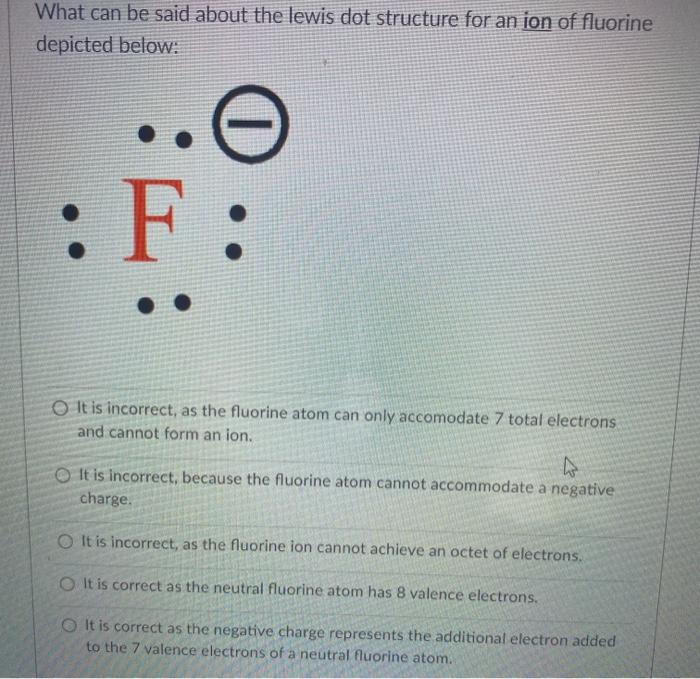
41 lewis electron dot diagram for fluoride ion
Hello everyone In this question we asked for the lewis electron dot diagram for fluoride ion on the periodic table. Flooring Is in group seven So it has ...1 answer · Top answer: In order for us to draw the electron dot diagram of fluoride, we must first determine the number of valence electrons of itFluorine can be seen in ... 1 answerUpon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas .
Non-valence electrons are not represented in Lewis structures. Lewis electron dot structure for fluoride ion.1 answer · Top answer: Hint: Lewis electron dot structures are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist ...
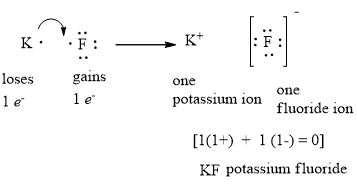
Lewis electron dot diagram for fluoride ion
A Lewis structure shows two fluorine atoms, each with three lone pairs of electrons,. 5. Rearrange the electrons of the outer atoms to make multiple bonds with ... Jan 7, 2017 · 1 answerFluorine is in Group 17 of the Periodic Table.................... And thus the neutral atom has 7 valence electrons. Of course the elemental ... Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in ...
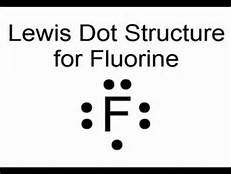
Lewis electron dot diagram for fluoride ion. And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. https://tse4.mm.bing.net/th?id= Upon reduction, the fluorine ...1 answer · 0 votes: Fluorine is in Group 17 of the Periodic Table.................... Explanation: And thus the neutral atom has 7 valence electrons. Of course the elemental ... 1 answerThe lewis electron dot diagram of fluorine must be able to represent its 7 valence electrons. Electron Dot Diagram of Fluorine. Thus, (b) 7 is... Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in ... Jan 7, 2017 · 1 answerFluorine is in Group 17 of the Periodic Table.................... And thus the neutral atom has 7 valence electrons. Of course the elemental ...
A Lewis structure shows two fluorine atoms, each with three lone pairs of electrons,. 5. Rearrange the electrons of the outer atoms to make multiple bonds with ...

Solved Chapter 6 Problem 13qp Solution Masteringchemistry Standalone Access Card For Basic Chemistry 3rd Edition Chegg Com

Draw The Electron Dot Structures For A H 2 S B F 2 Sarthaks Econnect Largest Online Education Community







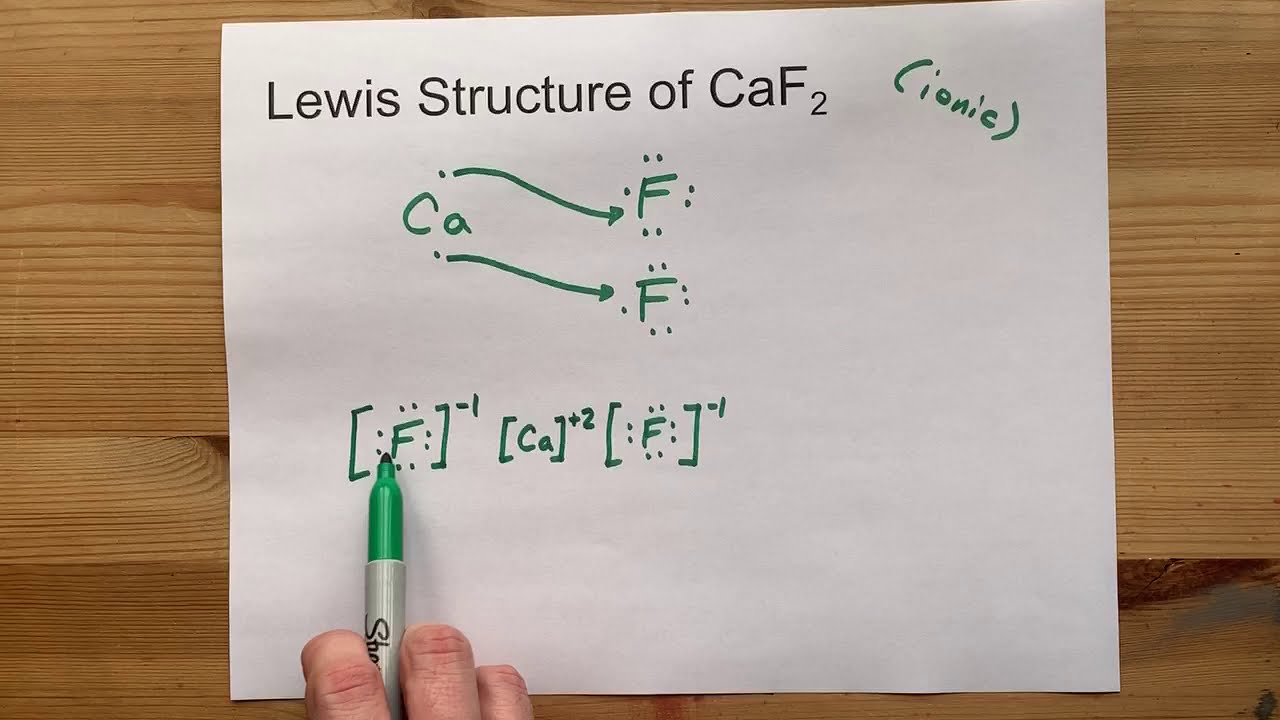



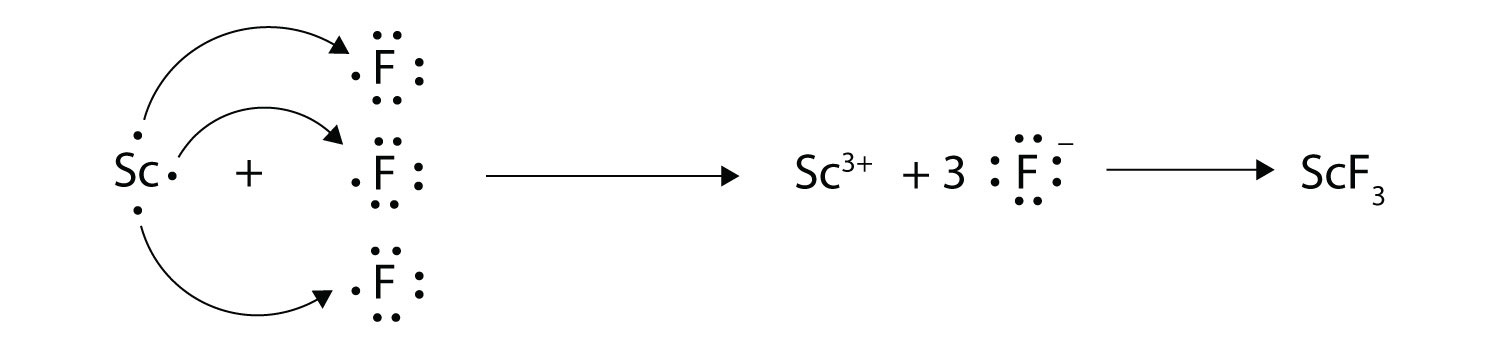
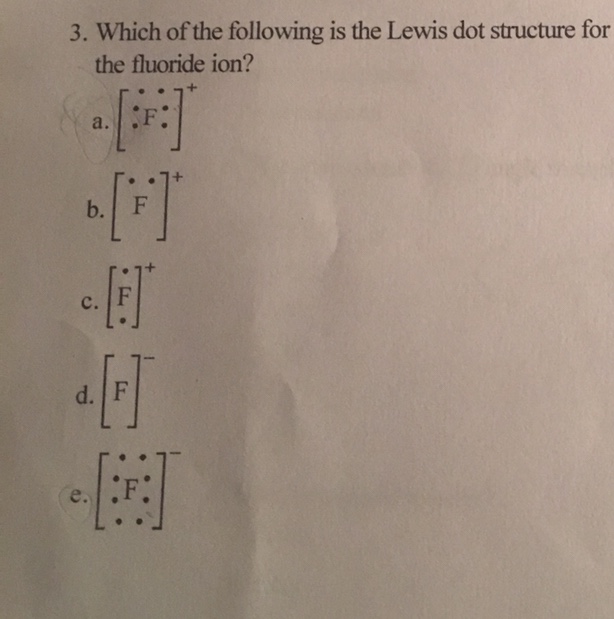

:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)











Comments
Post a Comment