41 hcl molecular orbital diagram
Hi /r/Chempros. Have you ever shed blood and tears on writing a script, only to find after a few weeks that something really similar had already been done? Have you ever created a specific tool but didn't really had the time or the right place to share it with your colleagues? Have you ever seen a really useful reddit post that you wish you had saved? I have, and after a quick exchange with our dear mod /u/wildfyr I've decided to post this thread. #Scope I would like for it to be a location ... Use molecular orbital theory to predict molecular geometry for simple triatomic systems • Rationalize molecular structure for several specific systems in terms of orbital overlap and bonding. HCl What would you predict the MO diagram for HCl to look like?
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
Hcl molecular orbital diagram
The Molecular Orbital Theory. Thermodynamics, PV Diagrams, Internal Energy, Heat, Work, Isothermal, Adiabatic, Isobaric, Physics. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Draw the molecular orbital energy-level diagram for the system. B Determine the total number of valence electrons in the He22+ ion. antibonding orbital at higher energy. The bonding in any diatomic molecule with two He atoms can be described using the following molecular orbital diagram
Hcl molecular orbital diagram. • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital. 430 8.4 Molecular Orbital Theory . ... . . This diagram shows just some of the interrelationships between chemistry and other fields. 3. The word "acid" is added as a second word. For example, when the gas HCl (hydrogen chloride) is dissolved in water, the solution is called... Molecular Orbital Theory. I'm having a lot of trouble with this stuff. So here, I basically ask, how do I draw a correlation diagram? Like, how do I know how many electrons to put in Why is it necessary to remove HCl? Won't it just stay in the water-HCl mixture when the 2-chloro-2-methylpropane molecule... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
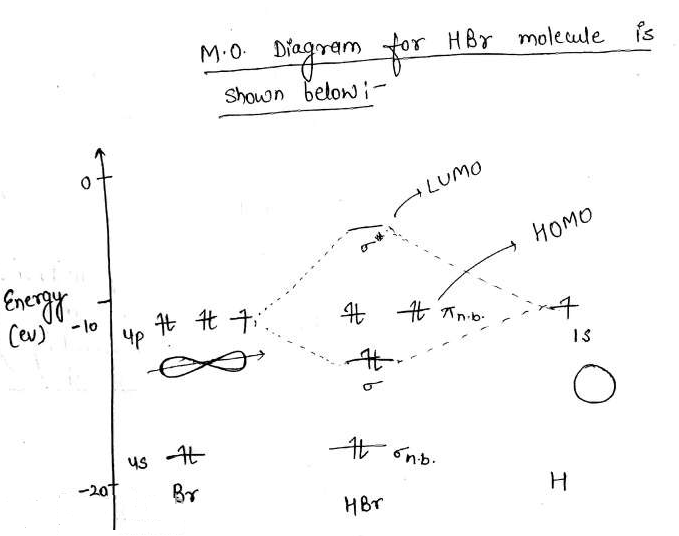
So, in MO diagrams for halogen acids (HF, HCl, etc.), the hydrogen's 1s orbital is shown to interact with a p_z_ orbital to form the molecular orbitals. However, 1s is symmetric to inversion, while p_z_ is anti-symmetric to inversion. From what I know orbitals with different symmetry cannot interact to form MO...I've been googling and combing through textbooks for an hour, what am I missing? edit: clarification, formatting. A molecular orbital diagram, or MO diagram for short, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.[1][2][3] A... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. Molecular orbital diagrams can be used to... calculate bond order. - Irradiation of a hydrogen (chlorine) molecule by UV light - causes the atom to separate - as promotion of one electron from bonding molecular orbital to antibonding molecular orbital - 1/2(Nb-Na) = 1/2(1-1) = O - Hydrogen...
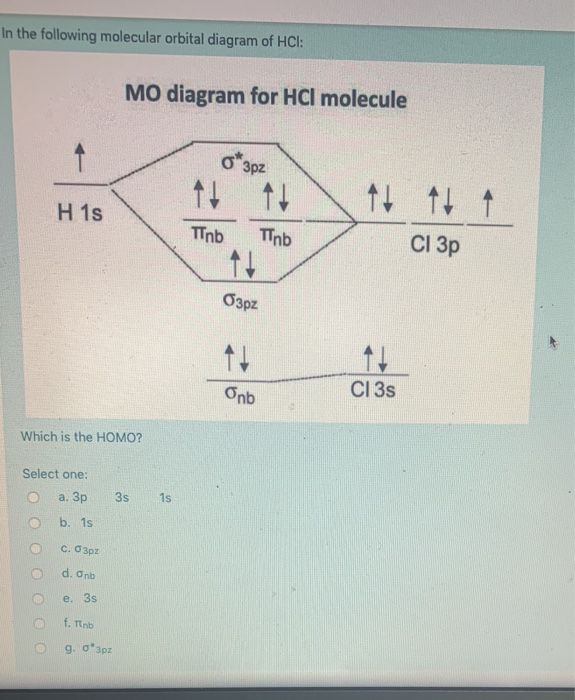
The bonding molecular orbital is fully filled with two electrons .The rest of the electrons remain in their atomic orbitals. Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and... HCL Molecular Orbital Diagram detailed information at Eduvark. Molecular orbital (MO) hypothesis can possibly be more quantitative. With it we can likewise get a photo of where the electrons are in the particle, as appeared in the picture at the privilege. Molecular Orbital Diagram Wikipedia The Free Encyclopedia Diagram Molecular Science Chemistry. 9 hours ago Using the molecular Orbital approach to describe Bonding in HCl, we can see from Figure 9. 31 molecular Orbital Energy - Level Diagram for HCl that 1 s Orbital of atomic... Line Chart. Diagram. Molecular Orbital Diagram of HCl.
Therefore, the HCL molecule has 8 pairs (1s, 2s, 2px,2py,2pz,3s,3px and 3py) of non-bonding (nb) electrons and one bonding (sigma) orbital having two electrons. The sigma antibonding orbital will be empty.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Q: Consider the reaction 4HCl (g) + O2 (g)= 2 H2O (g) + 2 Cl2 (g) Each molecular diagram represents an initial mixture of r.
Cyclobutadiene: Molecular Orbital Diagram, Antiaromaticity, and Structure. Previously, we've seen what the molecular orbitals of benzene look like, and that the fact that they are partially duplexed (or to use the proper nomenclature, "degenerate") helps to explain benzene's unusual stability. Let's flip the...
an orbital interaction diagram. Browse other questions tagged theoretical-chemistry quantum-chemistry molecular-orbital-theory or ask your own question.

Ps 1 C 1 F 1 C 2 F 2 Ps 2 C 1 F 1 C 2 F 2 Molecular Orbital Theory Lcao Mo Linear Combination Of Atomic Orbitals Add And Subtract Amplitudes Of Ppt Download
Hydrochloric acid or HCl is a very strong acid without any doubt. It is a colorless, pungent-smelling, chlorine-based acid-containing water. In simple words, the molecular orbital theory is the formation of molecular orbitals by the combination of atomic orbitals of the atoms in a molecule.
The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive...
Unlike earlier diagrams, only the molecular orbital energy levels for the molecules are shown here. For simplicity, the atomic orbital energy levels for the component atoms Use a qualitative molecular orbital energy-level diagram to predict the electron configuration, the bond order, and the number of...
Download scientific diagram | Schematic molecular orbital diagram for octahedrally coordinated Fe(II)-chloride complexes. The d → d transition is shown as a light double arrow and possible charge transfer transitions are shown as heavy arrows. This diagram and Fig. 2 were constructed based on...
Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Hydrogen chloride, HCl, is a diatomic molecule consisting of a hydrogen atom H and a chlorine atom Cl connected by a covalent single bond.
Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the One of the molecular orbitals in this molecule is constructed by adding the mathematical functions This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms.
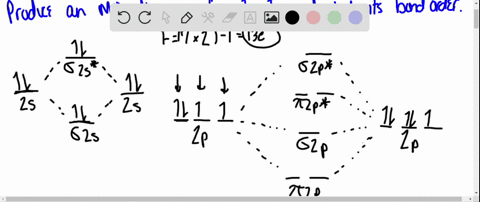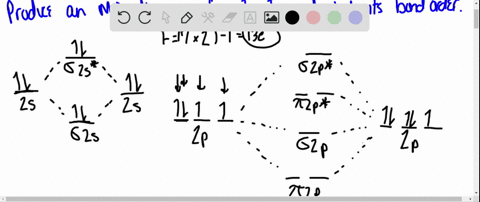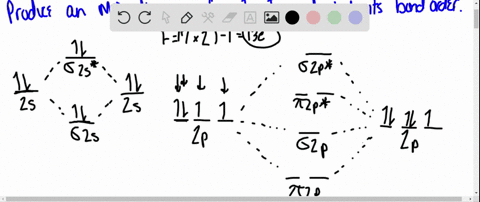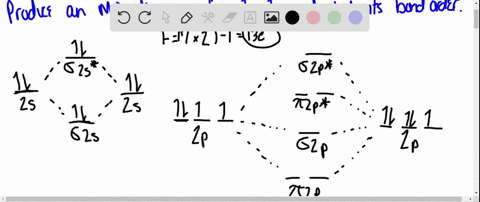
Solved Draw An Mo Energy Diagram For Hcl Predict The Bond Order And Make A Sketch Of The Lowest Energy Bonding Molecular Orbital
Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Draw the molecular orbital energy-level diagram for the system. B Determine the total number of valence electrons in the He22+ ion. antibonding orbital at higher energy. The bonding in any diatomic molecule with two He atoms can be described using the following molecular orbital diagram
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
The Molecular Orbital Theory. Thermodynamics, PV Diagrams, Internal Energy, Heat, Work, Isothermal, Adiabatic, Isobaric, Physics.

For The Reaction Co H2o 6 2 4cl Leftrightharpoons Cocl4 2 6h2o After Adding 12m Hcl What Would Be The Roles Of H Ions And Cl Ions Study Com
Chapter 1 Molecular Orbital Concepts A Concepts Of Mo Theory 1 Strong Covalent Bonds Consider The Pi Bond Of Ethene In Simple Molecular Orbital Terms The Qualitative Results Would Be The Same For Any Pi Or Sigma Bond Q The Overlap Of The Two
Solved Imagine A Group Of Four Hcl Molecules Draw Three Pictures Of This Group One In Each Of The Three States Of Matter Superimpose The Dipole Symbol 3a 4a On

Nanomaterials Free Full Text Sensing Behavior Of Metal Free Porphyrin And Zinc Phthalocyanine Thin Film Towards Xylene Styrene And Hcl Vapors In Planar Optical Waveguide Html















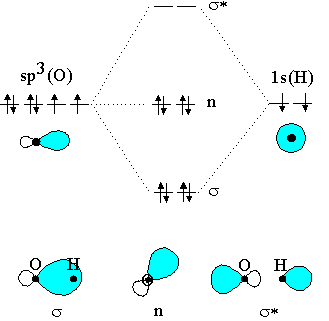



Comments
Post a Comment